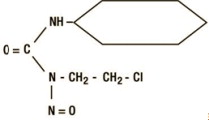
Gleostine
What is Gleostine (Lomustine)?
Approved To Treat
Related Clinical Trials
Summary: This phase II trial compares the safety, side effects and effectiveness of anti-lag-3 (relatlimab) and anti-PD-1 blockade (nivolumab) to standard of care lomustine for the treatment of patients with glioblastoma that has come back after a period of improvement (recurrent). Relatlimab is a monoclonal antibody that may interfere with the ability of tumor cells to grow and spread. A monoclonal antibo...
Summary: This is a phase 1 study evaluating the safety and feasibility of laser interstitial thermal therapy (LITT) followed by lomustine (CCNU) for recurrent glioblastoma in adults. The primary aim is to evaluate the safety of the combination of LITT plus lomustine based on the assessment of treatment-related adverse events and the feasibility of completing LITT + lomustine in the proposed timeframe. The ...
Summary: This is a prospective, multicenter, randomized controlled trial designed to evaluate the efficacy and safety of venetoclax-enhanced BUCY (Ven-BUCY) conditioning compared to the standard BUCY regimen in patients with high-risk acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS) undergoing allogeneic hematopoietic stem cell transplantation (allo-HSCT). Eligible participants aged 12 to 60...
Related Latest Advances
Brand Information
- 100 mg capsules (green/green)
- 40 mg capsules (white/green)
- 10 mg capsules (white/white)
- Delayed myelosuppression
- Risks of overdosage
- Pulmonary toxicity
- Secondary malignancies
- Hepatotoxicity
- Nephrotoxicity