Generic Name
Zolmitriptan
Brand Names
Zolmiptriptan, ZOMIG
FDA approval date: May 14, 2013
Classification: Serotonin-1b and Serotonin-1d Receptor Agonist
Form: Spray, Tablet
What is Zolmiptriptan (Zolmitriptan)?
Zolmitriptan tablets are indicated for the acute treatment of migraine with or without aura in adults. Limitations of Use Only use zolmitriptan tablets if a clear diagnosis of migraine has been established. If a patient has no response to zolmitriptan tablets treatment for the first migraine attack, reconsider the diagnosis of migraine before zolmitriptan tablets are administered to treat any subsequent attacks. Zolmitriptan tablets are not indicated for the prevention of migraine attacks. Safety and effectiveness of zolmitriptan tablets have not been established for cluster headache. Zolmitriptan tablets are a serotonin 1B/1D receptor agonist indicated for the acute treatment of migraine with or without aura in adults Limitations of Use: Use only after a clear diagnosis of migraine has been established Not indicated for the prophylactic therapy of migraine Not indicated for the treatment of cluster headache.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
zolmiptriptan (zolmitriptan)
1INDICATIONS AND USAGE
Zolmitriptan orally disintegrating tablets are indicated for the acute treatment of migraine with or without aura in adults.
Limitations of Use
- Only use zolmitriptan orally disintegrating tablets if a clear diagnosis of migraine has been established. If a patient has no response to zolmitriptan orally disintegrating tablets treatment for the first migraine attack, reconsider the diagnosis of migraine before zolmitriptan orally disintegrating tablets are administered to treat any subsequent attacks.
- Zolmitriptan orally disintegrating tablets are not indicated for the prevention of migraine attacks.
- Safety and effectiveness of zolmitriptan orally disintegrating tablets have not been established for cluster headache.
2DOSAGE FORMS AND STRENGTHS
2.5 mg Zolmitriptan Orally Disintegrating Tablets, USP: White to off-white, round tablets debossed with ‘F7’ on one side and plain on the other side.
5 mg Zolmitriptan Orally Disintegrating Tablets, USP: White to off-white, round tablets debossed with ‘F11’on one side and plain on the other side.
3CONTRAINDICATIONS
Zolmitriptan orally disintegrating tablets are contraindicated in patients with:
- Ischemic coronary artery disease (angina pectoris, history of myocardial infarction, or documented silent ischemia), other significant underlying cardiovascular disease, or
- Wolff-Parkinson-White Syndrome or arrhythmias associated with other cardiac accessory conduction pathway disorders [see
- History of stroke, transient ischemic attack (TIA), or history of hemiplegic or basilar migraine because these patients are at a higher risk of stroke [see
- Peripheral vascular disease (PVD) [see
- Ischemic bowel disease [see
- Uncontrolled hypertension [see
- Recent use (i.e., within 24 hours) of another 5-HT
- Concurrent administration of a monoamine oxidase (MAO)-A inhibitor or recent use of a MAO-A inhibitor (that is within 2 weeks) [see
- Known hypersensitivity to zolmitriptan orally disintegrating tablets (angioedema and anaphylaxis seen) [see
4ADVERSE REACTIONS
The following adverse reactions are described elsewhere in other sections of the prescribing information:
- Myocardial Ischemia, Myocardial Infarction, and Prinzmetal Angina [see
- Arrhythmias [
- Chest and or Throat, Neck and Jaw Pain/Tightness/Pressure [see
- Cerebrovascular Events [see
- Other Vasospasm Reactions [see
- Medication Overuse Headache [see
- Serotonin Syndrome [see
- Increase in Blood Pressure [see
- Risks in Patients with Phenylketonuria [see
4.1Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
In a long-term, open-label study where patients were allowed to treat multiple migraine attacks for up to 1 year, 8% (167 out of 2,058) withdrew from the trial because of adverse reaction.
The most common adverse reactions (≥ 5% and > placebo) in these trials were neck/throat/jaw pain, dizziness, paresthesia, asthenia, somnolence, warm/cold sensation, nausea, heaviness sensation, and dry mouth.
Table 1 lists the adverse reactions that occurred in ≥2% of the 2,074 patients in any one of the zolmitriptan 1 mg, 2.5 mg, or 5 mg dose groups in the controlled clinical trials of zolmitriptan in patients with migraines (Studies 1, 2, 3, 4, and 5) [see
Several of the adverse reactions appear dose related, notably paresthesia, sensation of heaviness or tightness in chest, neck, jaw, and throat, dizziness, somnolence and possibly asthenia and nausea.
Table 1: Adverse Reaction Incidence in Five Pooled Placebo-Controlled Migraine Clinical Trials
- *Only adverse reactions that were at least 2% more frequent in a zolmitriptan group compared to the placebo group are included.
There were no differences in the incidence of adverse reactions in controlled clinical trials in the following subgroups: gender, weight, age, use of prophylactic medications, or presence of aura. There were insufficient data to assess the impact of race on the incidence of adverse reactions.
4.1.1Less Common Adverse Reactions with Zolmitriptan Tablets:
In the paragraphs that follow, the frequencies of less commonly reported adverse clinical reactions are presented. Because the reports include reactions observed in open and uncontrolled studies, the role of zolmitriptan in their causation cannot be reliably determined. Furthermore, variability associated with adverse reaction reporting, the terminology used to describe adverse reactions, etc., limit the value of the quantitative frequency estimates provided. Adverse reaction frequencies were calculated as the number of patients who used zolmitriptan tablets and reported a reaction divided by the total number of patients exposed to zolmitriptan tablets (n=4,027). Reactions were further classified within body system categories and enumerated in order of decreasing frequency using the following definitions: infrequent adverse reactions (those occurring in 1/100 to 1/1,000 patients) and rare adverse reactions (those occurring in less than 1/1,000 patients).
General: Infrequent were allergic reactions.
Cardiovascular: Infrequent were arrhythmias, hypertension, and syncope. Rare was tachycardia.
Neurological: Infrequent were agitation, anxiety, depression, emotional lability and insomnia; Rare were amnesia, hallucinations, and cerebral ischemia.
Skin: Infrequent were pruritus, rash and urticaria.
Urogenital: Infrequent were polyuria, urinary frequency and urinary urgency.
4.1.2Adverse Reactions with Zolmitriptan Orally Disintegrating Tablets
The adverse reaction profile seen with zolmitriptan orally disintegrating tablets was similar to that seen with zolmitriptan tablets.
4.2Postmarketing Experience
The following adverse reactions were identified during post approval use of zolmitriptan. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The reactions enumerated include all except those already listed in the Clinical Trials Experience section above or the Warnings and Precautions section.
Hypersensitivity Reactions:
As with other 5-HT
5OVERDOSAGE
There is no experience with acute overdose of zolmitriptan. Clinical study subjects who received single 50 mg oral doses of zolmitriptan commonly experienced sedation.
There is no specific antidote to zolmitriptan. In cases of severe intoxication, intensive care procedures are recommended, including establishing and maintaining a patent airway, ensuring adequate oxygenation and ventilation, and monitoring and support of the cardiovascular system.
The elimination half-life of zolmitriptan is 3 hours [see
6DESCRIPTION
Zolmitriptan Orally Disintegrating Tablets, USP contain zolmitriptan, which is a selective 5-hydroxytryptamine
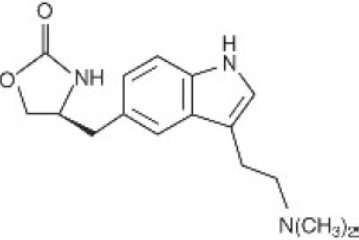
The molecular formula is C
Zolmitriptan Orally Disintegrating Tablets, USP are available as 2.5 mg and 5 mg white uncoated tablets for oral administration. The orally disintegrating tablets contain aspartame [see
7HOW SUPPLIED/STORAGE AND HANDLING
ZolmitriptanOrally Disintegrating Tablets, USP 2.5 mg - White to off-white, round tablets debossed with ‘F7’ on one side and plain on the other side are supplied in cartons containing a blister pack of 6 unit-dose tablets (NDC 68462-499-76)
Zolmitriptan Orally Disintegrating Tablets, USP 5 mg - White to off-white, round tablets debossed with ‘F11’on one side and plain on the other side are supplied in cartons containing a blister pack of 3 unit-dose tablets (NDC 68462-500-33)
Store zolmitriptan orally disintegrating tablets at 20°C to 25°C (68°F to 77°F); [see USP Controlled Room Temperature]. Protect from light and moisture.
8PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Myocardial Ischemia and/or Infarction, Prinzmetal’s Angina, Other Vasospastic Reactions, and Cerebrovascular Events
Inform patients that zolmitriptan may cause serious cardiovascular adverse reactions such as myocardial infarction or stroke, which may result in hospitalization and even death. Although serious cardiovascular reactions can occur without warning symptoms, instruct patients to be alert for the signs and symptoms of chest pain, shortness of breath, weakness, slurring of speech, and instruct them to ask for medical advice when observing any indicative sign or symptoms. Instruct patients to seek medical advice if they have symptoms of other vasospastic reactions [see
Medication Overuse Headache
Inform patients that use of drugs to treat acute migraines for 10 or more days per month may lead to an exacerbation of headache, and encourage patients to record headache frequency and drug use (e.g., by keeping a headache diary) [see
Serotonin Syndrome
Inform patients about the risk of serotonin syndrome with the use of zolmitriptan or other triptans, particularly during combined use with selective serotonin reuptake inhibitors (SSRIs) or serotonin norepinephrine reuptake inhibitors (SNRIs) [see
Pregnancy
- Advise patients to notify their healthcare provider if they are pregnant or plan to become pregnant.
Lactation
Advise patients to notify their healthcare provider if they are breastfeeding or plan to breastfeed [
Handling of Zolmitriptan Orally Disintegrating Tablets
Inform patients not to break zolmitriptan orally disintegrating tablets. Inform patients that the orally disintegrating tablet is packaged in a blister. Instruct patients not to remove the orally disintegrating tablet from the blister until just prior to dosing. Instruct patients that prior to dosing, peel open the blister pack and place the orally disintegrating tablet on the tongue, where it will dissolve and be swallowed with the saliva [see
Patients with Phenylketonuria
Inform patients with phenylketonuria (PKU) that zolmitriptan orally disintegrating tablets contain phenylalanine (a component of aspartame) [see
Manufactured by:
Glenmark Pharmaceuticals Limited
Colvale-Bardez, Goa 403513, India
Manufactured for:

Glenmark Pharmaceuticals Inc., USA
Mahwah, NJ 07430
Questions? 1 (888) 721-7115
www.glenmarkpharma-us.com
July 2022
9Patient Information
Zolmitriptan (ZOLE-mi-TRIP-tan)
Orally Disintegrating Tablets, USP
Orally Disintegrating Tablets, USP
Please read this information before you start taking zolmitriptan orally disintegrating tablets and each time you renew your prescription just in case anything has changed. Remember, this summary does not take the place of discussions with your doctor. You and your doctor should discuss zolmitriptan orally disintegrating tablets when you start taking your medication and at regular checkups.
What are zolmitriptan orally disintegrating tablets?
Zolmitriptan orally disintegrating tablets are a prescription medication used to treat migraine headaches in adults. Zolmitriptan orally disintegrating tablets are not for other types of headaches. The safety and efficacy of zolmitriptan orally disintegrating tablets in patients under 18 have not been established.
What is a Migraine Headache?
Migraine is an intense, throbbing headache. You may have pain on one or both sides of your head. You may have nausea and vomiting, and be sensitive to light and noise. The pain and symptoms of a migraine headache can be worse than a common headache. Some women get migraines around the time of their menstrual period. Some people have visual symptoms before the headache, such as flashing lights or wavy lines, called an aura.
How do zolmitriptan orally disintegrating tablets work?
Treatment with zolmitriptan orally disintegrating tablets reduces swelling of blood vessels surrounding the brain. This swelling is associated with the headache pain of a migraine attack. Zolmitriptan orally disintegrating tablets block the release of substances from nerve endings that cause more pain and other symptoms like nausea, and sensitivity to light and sound. It is thought that these actions contribute to relief of your symptoms by zolmitriptan orally disintegrating tablets.
Who should not take zolmitriptan orally disintegrating tablets?
Do not take zolmitriptan orally disintegrating tablets if you:
- Have heart disease or a history of heart disease
- Have uncontrolled high blood pressure
- Have hemiplegic or basilar migraine (if you are not sure about this, ask your doctor)
- Have or had a stroke or problems with your blood circulation
- Have serious liver problems
- Have taken any of the following medicines in the last 24 hours: other “triptans” like almotriptan (AXERT
- Have taken monoamine oxidase (MAO) inhibitors such as phenelzine sulfate (NARDIL
- Are allergic to zolmitriptan orally disintegrating tablets or any of its ingredients. The active ingredient is zolmitriptan. The inactive ingredients are listed at the end of this leaflet.
Tell your doctor about all the medicines you take or plan to take, including prescription and non-prescription medicines, supplements, and herbal remedies.
Tell your doctor if you are sensitive to phenylalanine, which can be found in the artificial sweetener aspartame. Zolmitriptan orally disintegrating tablets contain phenylalanine.
Tell your doctor if you are taking selective serotonin reuptake inhibitors (SSRIs) or serotonin norepinephrine reuptake inhibitors (SNRIs), two types of drugs for depression or other disorders. Common SSRIs are CELEXA
Tell your doctor if you know that you have any of the following: risk factors for heart disease like high cholesterol, diabetes, smoking, obesity (overweight), menopause, or a family history of heart disease or stroke.
Tell your doctor if you are pregnant or plan to become pregnant. It is not known if zolmitriptan will harm your unborn baby.
Tell your doctor if you are breastfeeding or plan to breastfeed. It is not known if zolmitriptan passes into your breast milk. Talk to your doctor about the best way to feed your baby while using zolmitriptan orally disintegrating tablets.
How should I take zolmitriptan orally disintegrating tablets?
- Take zolmitriptan orally disintegrating tablets exactly as your doctor tells you to take it. Your doctor will tell you how many zolmitriptan orally disintegrating tablets to take and when to take them.
- If you take zolmitriptan orally disintegrating tablets, do not remove the tablet from the blister pack until you are ready to take your medicine.
- You do not need to take any liquids with your zolmitriptan orally disintegrating tablets.
- Take zolmitriptan orally disintegrating tablets whole.
- Place zolmitriptan orally disintegrating tablets on your tongue, where it will dissolve.
- Safely throw away any unused tablets or pieces of tablets that have been removed from the blister packaging.
- If your headache comes back after your first dose, you may take a second dose any time after 2 hours of taking the first dose. For any attack where the first dose did not work, do not take a second dose without talking with your doctor. Do not take more than a total of 10 mg of zolmitriptan (tablets or spray combined) in any 24 hour period. If you take too much medicine, contact your doctor, hospital emergency department, or poison control center right away.
What are the possible side effects of zolmitriptan orally disintegrating tablets?
Zolmitriptan orally disintegrating tablets are generally well tolerated. As with any medicine, people taking zolmitriptan orally disintegrating tablets may have side effects. The side effects are usually mild and do not last long.
The most common side effects of zolmitriptan orally disintegrating tablets are:
- pain, pressure or tightness in the neck, throat or jaw
- dizziness
- tingling or other abnormal sensations
- tiredness
- drowsiness
- feeling warm or cold
- nausea
- feeling of tightness or heaviness in other areas of the body
- dry mouth
In very rare cases, patients taking triptans may experience serious side effects, such as heart attacks, high blood pressure, stroke, or serious allergic reactions. Extremely rarely, patients have died.
- severe tightness, pain, pressure or heaviness in your chest, throat, neck, or jaw
- shortness of breath or wheezing
- sudden or severe stomach pain
- hives; tongue, mouth, or throat swelling
- problems seeing
- unusual weakness or numbness
Some people may have a reaction called serotonin syndrome, which can be life-threatening, when they use zolmitriptan orally disintegrating tablets. In particular, this reaction may occur when they use zolmitriptan orally disintegrating tablets together with certain types of antidepressants known as SSRIs or SNRIs. Symptoms may include mental changes (hallucinations, agitation, coma), fast heartbeat, changes in blood pressure, high body temperature or sweating, tight muscles, trouble walking, nausea, vomiting, and diarrhea. Call your doctor immediately if you have any of these symptoms after taking zolmitriptan orally disintegrating tablets.
This is not a complete list of side effects. Talk to your doctor if you develop any symptoms that concern you.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
What to do in case of an overdose?
Call your doctor or poison control center or go to the nearest hospital emergency room.
General advice about zolmitriptan orally disintegrating tablets
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not use zolmitriptan orally disintegrating tablets for a condition for which they were not prescribed. Do not give zolmitriptan orally disintegrating tablets to other people, even if they have the same symptoms as you. People may be harmed if they take medicines that have not been prescribed for them.
This leaflet summarizes the most important information about zolmitriptan orally disintegrating tablets. If you would like more information about zolmitriptan orally disintegrating tablets, talk to your doctor. You can ask your doctor or pharmacist for information on zolmitriptan orally disintegrating tablets that is written for healthcare professionals.
You can also call 1 (888) 721-7115 or visit our website at www.glenmarkpharma-us.com.
What are the ingredients in zolmitriptan orally disintegrating tablets?
Active ingredient: zolmitriptan
Inactive ingredients: aspartame, colloidal silicon dioxide, crospovidone, magnesium stearate, mannitol, microcrystalline cellulose, peppermint flavor and talc. The peppermint flavor contains corn starch.
Store zolmitriptan orally disintegrating tablets at 20°C to 25°C (68°F to 77°F); [see USP Controlled Room Temperature] and away from children.
Protect from light and moisture. Discard when expired.
Other brands mentioned are trademarks of their respective owners and are not trademarks of Glenmark Pharmaceuticals Limited. The makers of these brands are not affiliated with Glenmark Pharmaceuticals Limited or its products.
Manufactured by:
Glenmark Pharmaceuticals Limited
Colvale-Bardez, Goa 403513, India
Manufactured for:

Glenmark Pharmaceuticals Inc., USA
Mahwah, NJ 07430
Questions? 1 (888) 721-7115
www.glenmarkpharma-us.com
July 2022
10Package/Label Display Panel
NDC 68462-499-76
Zolmitriptan Orally Disintegrating Tablets, 2.5 mg
Carton Label – 6 unit-dose tablets
Zolmitriptan Orally Disintegrating Tablets, 2.5 mg
Carton Label – 6 unit-dose tablets

11Package/Label Display Panel
NDC 68462-500-33
Zolmitriptan Orally Disintegrating Tablets, 2.5 mg
Carton Label – 3 unit-dose tablets
Zolmitriptan Orally Disintegrating Tablets, 2.5 mg
Carton Label – 3 unit-dose tablets

