Xarelto
What is Xarelto (Rivaroxaban)?
Approved To Treat
Related Clinical Trials
Summary: This study is a prospective, multicenter, randomized controlled trial of the FlowTriever System plus anticoagulation compared to anticoagulation alone for intermediate-risk acute PE.
Summary: The goal of this pilot study is to assess enrollment feasibility of a randomized trial of direct oral anticoagulant and high-intensity statin therapy versus usual care in patients with Myocardial Injury after Noncardiac Surgery (MINS). The primary aims of this study are to assess feasibility, study drug adherence, and optimize study design (entry criteria, study endpoints, sample size calculation,...
Summary: This study is a prospective, multicenter, randomized controlled trial of an interventional strategy using the ClotTriever System to achieve and maintain vessel patency (ClotTriever Intervention Arm) versus conservative medical management using anticoagulation therapy alone (Conservative Medical Management Arm) in the treatment of subjects with symptomatic unilateral iliofemoral DVT. The study will...
Related Latest Advances
Brand Information
- 2.5 mg tablets: Round, light yellow, and film-coated with a triangle pointing down above a "2.5" marked on one side and "Xa" on the other side
- 10 mg tablets: Round, light red, biconvex and film-coated with a triangle pointing down above a "10" marked on one side and "Xa" on the other side
- 15 mg tablets: Round, red, biconvex, and film-coated with a triangle pointing down above a "15" marked on one side and "Xa" on the other side
- 20 mg tablets: Triangle-shaped, dark red, and film-coated with a triangle pointing down above a "20" marked on one side and "Xa" on the other side
- For oral suspension: white to off-white granules; once reconstituted, provide flavored white to off-white opaque liquid with a concentration of 1 mg/mL.
- active pathological bleeding
- severe hypersensitivity reaction to XARELTO (e.g., anaphylactic reactions)
- Increased Risk of Stroke After Discontinuation in Nonvalvular Atrial Fibrillation
- Bleeding Risk
- Spinal/Epidural Hematoma
- 2.5 mg tablets are round, light yellow, and film-coated with a triangle pointing down above a "2.5" marked on one side and "Xa" on the other side. The tablets are supplied in the packages listed:
- 10 mg tablets are round, light red, biconvex film-coated tablets marked with a triangle pointing down above a "10" on one side, and "Xa" on the other side. The tablets are supplied in the packages listed:
- 15 mg tablets are round, red, biconvex film-coated tablets with a triangle pointing down above a "15" marked on one side and "Xa" on the other side. The tablets are supplied in the packages listed:
- 20 mg tablets are triangle-shaped, dark red film-coated tablets with a triangle pointing down above a "20" marked on one side and "Xa" on the other side. The tablets are supplied in the packages listed:
- Starter Pack for treatment of deep vein thrombosis and treatment of pulmonary embolism:
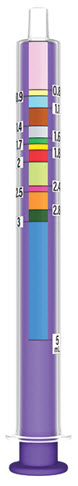
- XARELTO suspension is for oral use only.
- Give XARELTO to your child exactly as prescribed by your doctor. The adult caregiver should give the dose. If you have questions, contact your doctor or pharmacist for more information on giving a dose.
- Only use the oral dosing syringe provided with XARELTO oral suspension.Contact your doctor or pharmacist if the oral dosing syringe is missing, lost or damaged.
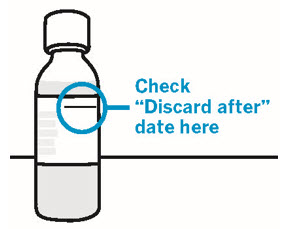
 Storage information
Storage information
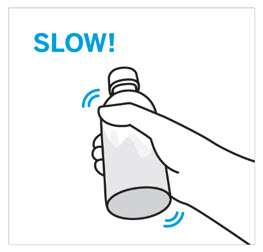
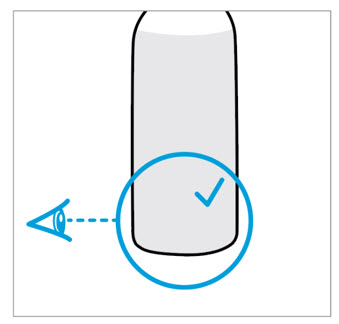
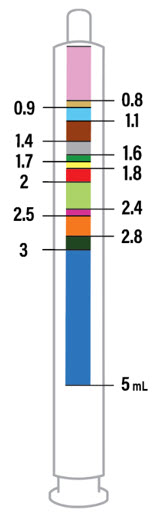
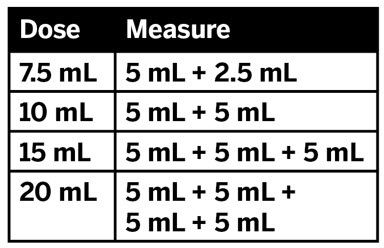
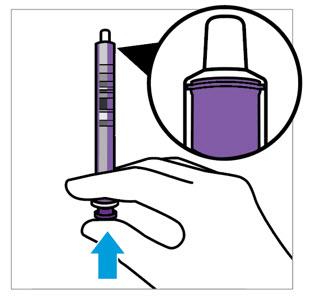
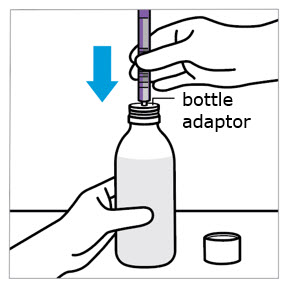
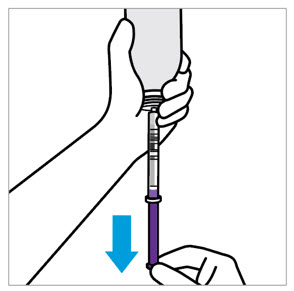
 CAUTION:
CAUTION: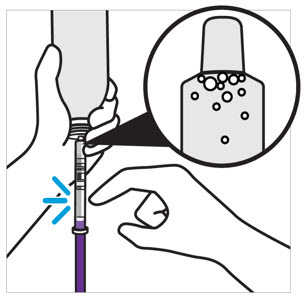
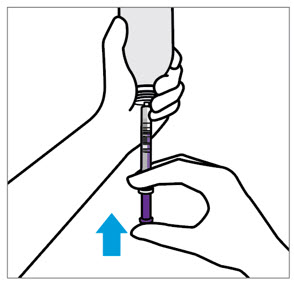
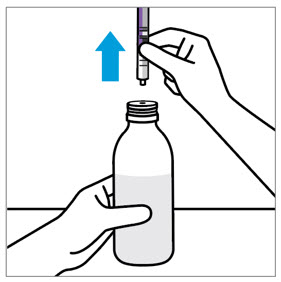
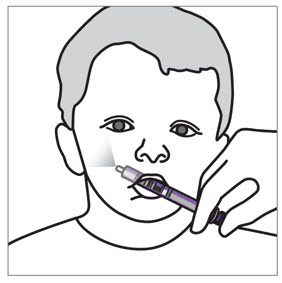
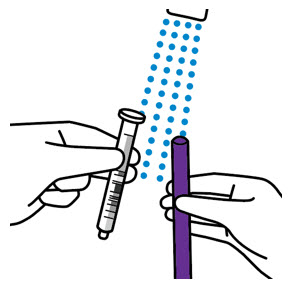
- Throw the XARELTO bottle away in your household trash.
- Throw away any used oral dosing syringe with the opening of a new XARELTO bottle.
- Do not pour XARELTO suspension down the drain(for example: sink, toilet, shower or tub).
- Do notrecycle the bottle.





- Must reconstitute before
- Counsel caregiver on proper use.





