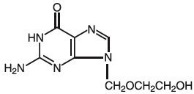
Acyclovir
What is Zovirax (Acyclovir)?
Related Clinical Trials
Summary: This is a Phase 1 single-arm open-label study of letermovir in neonates with symptomatic congenital Cytomegalovirus (CMV) disease. There will be two groups enrolled. Group 1 will be comprised of 4 subjects. Following documentation study inclusion and signing of informed consent, Group 1 subjects will receive one dose of oral letermovir (Study Day 0), using the dose bands. A full pharmacokinetics (...
Summary: To learn if the combination of pirtobrutinib (also called LOXO-305), venetoclax, and obinutuzumab is safe and effective when given to patients with chronic lymphocytic leukemia (CLL) or Richter transformation (RT) who have not previously received treatment.
Summary: This study is being done to compare the effectiveness of de novo Letermovir versus valganciclovir in preventing the development of cytomegalovirus viremia or symptomatic disease in African American kidney transplant recipients within the first year after transplantation. There are two arms in the study: Arm 1: Prophylaxis: This group includes freshly transplanted high risk (CMV D+/R-) African Amer...
Related Latest Advances
Brand Information
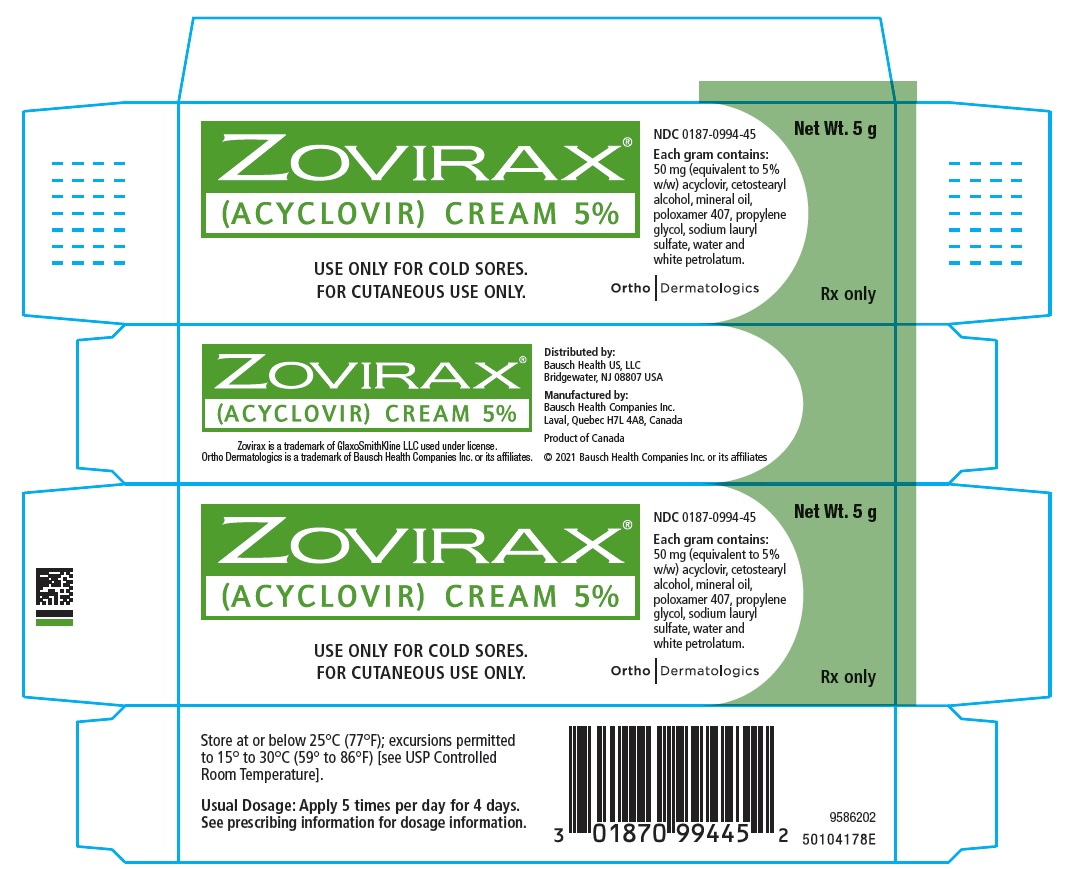
- ZOVIRAX Cream is a prescription medicine used to treat cold sores (herpes labialis) that are recurring in adults and children 12 years of age and older, and who have normal immune systems.
- ZOVIRAX Cream is not a cure for cold sores.
- become sick very easily (have a weak immune system).
- are pregnant or plan to become pregnant. It is not known if ZOVIRAX Cream will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if ZOVIRAX Cream passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you use ZOVIRAX Cream.
- Use ZOVIRAX Cream exactly as your healthcare provider tells you to use it.
- Use ZOVIRAX Cream as soon as you have the first symptoms of a cold sore such as itching, redness, burning or tingling, or when the cold sore appears.
- Wash your hands with soap and water before and after applying ZOVIRAX Cream.
- The affected area should be clean and dry before applying ZOVIRAX Cream.
- Apply ZOVIRAX Cream to the affected area 5 times each day for 4 days, including the outer edge.
- You should not apply other skin products to the affected area during treatment with ZOVIRAX Cream.
- Avoid unnecessary rubbing of the cold sore because this may cause the cold sore to spread to other areas around your mouth or make your cold sore worse.
- Store ZOVIRAX Cream at room temperature between 68° to 77°F (20° to 25°C).
Inactive ingredients: cetostearyl alcohol, mineral oil, poloxamer 407, propylene glycol, sodium lauryl sulfate, water, and white petrolatum
Bridgewater, NJ 08807 USA
Manufactured by: Bausch Health Companies Inc.
Laval, Quebec H7L 4A8, Canada
(ACYCLOVIR) CREAM 5%
Net Wt. 5 g
50 mg (equivalent to 5%
w/w) acyclovir, cetostearyl
alcohol, mineral oil,
poloxamer 407, propylene
glycol, sodium lauryl
sulfate, water and
white petrolatum.
FOR CUTANEOUS USE ONLY.