Generic Name
Doxepin
Brand Names
Zonalon, Silenor, Prudoxin
FDA approval date: May 13, 1986
Classification: Tricyclic Antidepressant
Form: Cream, Tablet, Capsule, Solution
What is Zonalon (Doxepin)?
Doxepin Hydrochloride Capsules, USP are recommended for the treatment of: 1. Psychoneurotic patients with depression and/or anxiety. 2. Depression and/or anxiety associated with alcoholism . 3. Depression and/or anxiety associated with organic disease . 4. Psychotic depressive disorders with associated anxiety including involutional depression and manic-depressive disorders. The target symptoms of psychoneurosis that respond particularly well to doxepin hydrochloride capsules include anxiety, tension, depression, somatic symptoms and concerns, sleep disturbances, guilt, lack of energy, fear, apprehension and worry. Clinical experience has shown that doxepin hydrochloride capsules is safe and well tolerated even in the elderly patient. Owing to lack of clinical experience in the pediatric population, doxepin is not recommended for use in children under 12 years of age.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
Doxepin Solution for Alleviation of Stubborn Breakthrough Pain Induced by Swallowing in Patient Receiving Radiotherapy for Nasopharyngeal Carcinoma: A Multicenter, Randomized, Controlled, Double-Blind Clinical Trial
Summary: The goal of this clinical trial is to explore the effectiveness and adverse reactions of doxepin solution spray for alleviation of stubborn breakthrough pain induced by swallowing in patients receiving radiotherapy for nasopharyngeal carcinoma.
Related Latest Advances
Brand Information
Zonalon (doxepin hydrochloride)
1DESCRIPTION
Zonalon
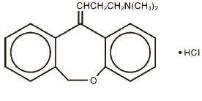
Doxepin hydrochloride, USP is one of a class of agents known as dibenzoxepin tricyclic antidepressant compounds. It is an isomeric mixture of N,N-dimethyldibenz[
Zonalon
2CLINICAL PHARMACOLOGY
Although doxepin HCl does have H
Once absorbed into the systemic circulation, doxepin undergoes hepatic metabolism that results in conversion to pharmacologically-active desmethyldoxepin. Further glucuronidation results in urinary excretion of the parent drug and its metabolites. Desmethyldoxepin has a half-life that ranges from 28 to 52 hours and is not affected by multiple dosing. Plasma levels of both doxepin and desmethyldoxepin are highly variable and are poorly correlated with dosage. Wide distribution occurs in body tissues including lungs, heart, brain, and liver. Renal disease, genetic factors, age, and other medications affect the metabolism and subsequent elimination of doxepin. (See
3INDICATIONS AND USAGE
Zonalon
4CONTRAINDICATIONS
Because doxepin HCl has an anticholinergic effect and because significant plasma levels of doxepin are detectable after topical Zonalon
Zonalon
5WARNINGS
Drowsiness occurs in over 20% of patients treated with Zonalon
The sedating effects of alcoholic beverages, antihistamines, and other CNS depressants may be potentiated when Zonalon
If excessive drowsiness occurs it may be necessary to reduce the frequency of applications, the amount of cream applied, and/or the percentage of body surface area treated, or discontinue the drug. However, the efficacy with reduced frequency of applications has not been established.
Keep this product away from the eyes.
6OVERDOSAGE
Deaths may occur from overdosage with this class of drugs. As the management is complex and changing, it is recommended that the physician contact a poison control center for current information on treatment. Signs and symptoms of toxicity develop rapidly after tricyclic antidepressant overdose; therefore, hospital monitoring is required as soon as possible.
6.1Manifestations
Should overdosage with topical application of Zonalon
Other signs of overdose may include: confusion, disturbed concentration, transient visual hallucinations, dilated pupils, agitation, hyperactive reflexes, stupor, drowsiness, muscle rigidity, vomiting, hypothermia, hyperpyrexia, or any of the symptoms listed under
7DOSAGE AND ADMINISTRATION
A thin film of Zonalon
The risk for sedation may increase with greater body surface area application of Zonalon
Occlusive dressings may increase the absorption of most topical drugs; therefore, occlusive dressings should not be utilized with Zonalon
8HOW SUPPLIED
ZONALON
NDC 0378-8123-30
NDC 0378-8123-45
Store below 27°C (80°F).
Manufactured for:
Manufactured by:
ZONALON is a registered trademark of Delcor Asset Corporation, a Mylan Company
©2017 Mylan Pharmaceuticals Inc.
DPT:ZONA:R1
140900-0617
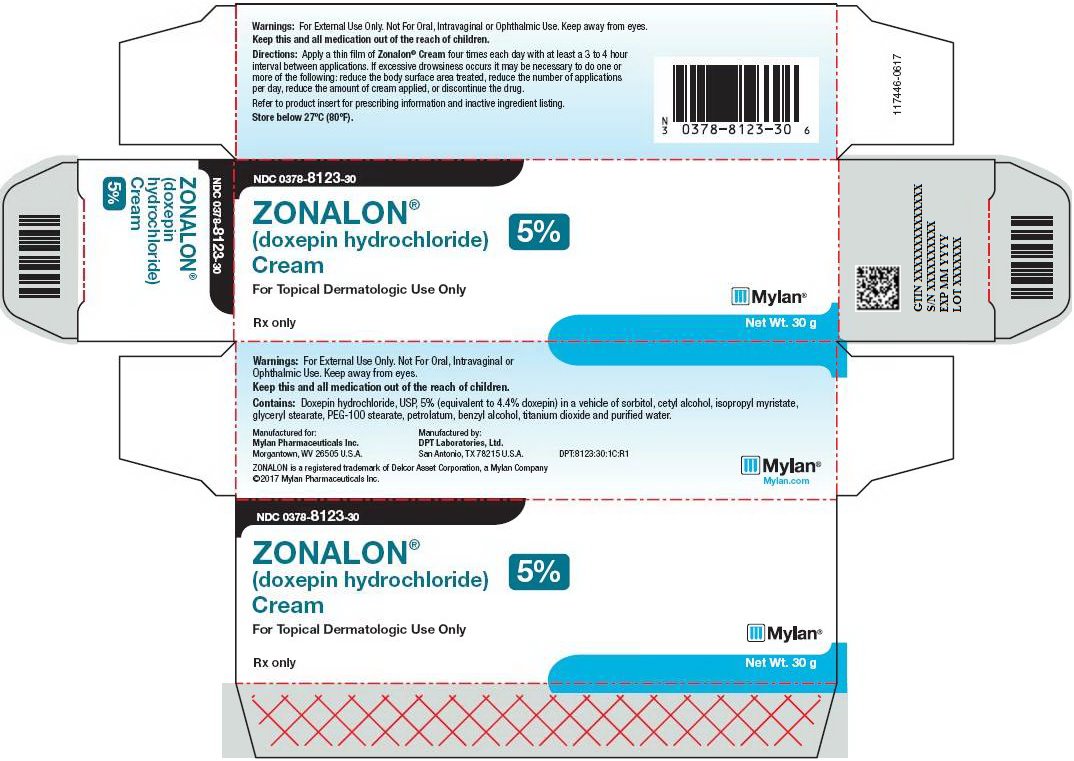
9PRINCIPAL DISPLAY PANEL – 5%
NDC 0378-8123-30
ZONALON
(doxepin hydrochloride)
Cream 5%
(doxepin hydrochloride)
Cream 5%
For Topical Dermatologic Use Only
Rx only Net Wt. 30 g
Warnings: For External Use Only. Not For Oral, Intravaginal or Ophthalmic Use. Keep away from eyes.
Keep this and all medication out of the reach of children.
Directions: Apply a thin film of Zonalon four times each day with at least a 3 to 4 hour
interval between applications. If excessive drowsiness occurs it may be necessary to do one or
more of the following: reduce the body surface area treated, reduce the number of applications
per day, reduce the amount of cream applied, or discontinue the drug.
interval between applications. If excessive drowsiness occurs it may be necessary to do one or
more of the following: reduce the body surface area treated, reduce the number of applications
per day, reduce the amount of cream applied, or discontinue the drug.
Refer to product insert for prescribing information and inactive ingredient listing.
Store below 27ºC (80ºF).
Contains: Doxepin hydrochloride, USP, 5% (equivalent to 4.4% doxepin) in a vehicle of sorbitol, cetyl alcohol, isopropyl myristate, glyceryl stearate, PEG-100 stearate, petrolatum, benzyl alcohol, titanium dioxide and purified water.
Manufactured for:
Manufactured by:
DPT:8123:30:1C:R1
ZONALON is a registered trademark of Delcor Asset Corporation, a Mylan Company
©2017 Mylan Pharmaceuticals Inc.