Generic Name
Tolvaptan
Brand Names
Samsca, Jynarque
FDA approval date: May 19, 2009
Classification: Vasopressin V2 Receptor Antagonist
Form: Tablet, Kit
What is Samsca (Tolvaptan)?
Tolvaptan tablets are indicated for the treatment of clinically significant hypervolemic and euvolemic hyponatremia , including patients with heart failure and Syndrome of Inappropriate Antidiuretic Hormone . Limitations of Use Patients requiring intervention to raise serum sodium urgently to prevent or to treat serious neurological symptoms should not be treated with tolvaptan tablets. It has not been established that raising serum sodium with tolvaptan tablets provides a symptomatic benefit to patients. Tolvaptan tablets are a selective vasopressin V 2 -receptor antagonist indicated for the treatment of clinically significant hypervolemic and euvolemic hyponatremia , including patients with heart failure and Syndrome of Inappropriate Antidiuretic Hormone Limitations of Use: Patients requiring intervention to raise serum sodium urgently to prevent or to treat serious neurological symptoms should not be treated with tolvaptan tablets It has not been established that tolvaptan tablets provides a symptomatic benefit to patients
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
A Phase 3b Multicenter Open-label Trial of the Safety, Tolerability, and Efficacy of Tolvaptan in Infants and Children 28 Days to Less Than 12 Weeks of Age With Autosomal Recessive Polycystic Kidney Disease (ARPKD)
Summary: The primary objective of this study is to evaluate the effect of tolvaptan on the need for renal replacement therapy in pediatric subjects with autosomal recessive polycystic kidney disease (ARPKD)
A Phase 3b Multicenter Open-label Trial of the Safety, Tolerability, and Efficacy of Tolvaptan in Infants and Children 28 Days to Less Than 18 Years of Age With Autosomal Recessive Polycystic Kidney Disease (ARPKD)
Summary: To evaluate the safety of tolvaptan in pediatric subjects with ARPKD
Canadian Observational Cohort Study of the Real-life Assessment of Tolvaptan (JINARC™) in Autosomal Dominant Polycystic Kidney Disease (ADPKD)
Summary: This study is part of the Health Canada approval requirement for JINARC™ (tolvaptan) and is an observational, non-interventional study (NIS) describing the impact of tolvaptan on ADPKD-related burden of illness as measured with a set of Patient Reported Outcome (PRO) Questionnaires. The study is also describing the time to renal replacement therapy (RRT), such as dialysis and transplantation, and ...
Related Latest Advances
Brand Information
SAMSCA (tolvaptan)
1INDICATIONS AND USAGE
SAMSCA
2DOSAGE FORMS AND STRENGTHS
SAMSCA is available in the following dosage forms and strengths:
- 15 mg tablets are non-scored, blue, triangular, shallow-convex, debossed with "OTSUKA" and "15" on one side.
- 30 mg tablets are non-scored, blue, round, shallow-convex, debossed with "OTSUKA" and "30" on one side.
3CONTRAINDICATIONS
SAMSCA is contraindicated in the following conditions:
- Patients with autosomal dominant polycystic kidney disease (ADPKD) outside of FDA-approved REMS
- Unable to sense or respond to thirst
- Hypovolemic hyponatremia
- Taking strong CYP3A inhibitors
- Anuria
- Hypersensitivity (e.g., anaphylactic shock, rash generalized) to tolvaptan or any components of the product
4OVERDOSAGE
Single oral doses up to 480 mg (8 times the maximum recommended daily dose) and multiple doses up to 300 mg once daily for 5 days have been well tolerated in studies in healthy subjects. There is no specific antidote for tolvaptan intoxication. The signs and symptoms of an acute overdose can be anticipated to be those of excessive pharmacologic effect: a rise in serum sodium concentration, polyuria, thirst, and dehydration/hypovolemia.
No mortality was observed in rats or dogs following single oral doses of 2000 mg/kg (maximum feasible dose). A single oral dose of 2000 mg/kg was lethal in mice, and symptoms of toxicity in affected mice included decreased locomotor activity, staggering gait, tremor and hypothermia.
In patients with suspected SAMSCA overdosage, assessment of vital signs, electrolyte concentrations, ECG and fluid status are recommended. Continue replacement of water and electrolytes until aquaresis abates.
Dialysis may not be effective in removing tolvaptan because of its high binding affinity for human plasma protein (>98%).
5DESCRIPTION
SAMSCA contains tolvaptan, a selective vasopressin V
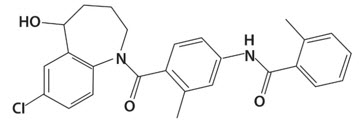
Inactive ingredients include corn starch, hydroxypropyl cellulose, lactose monohydrate, low-substituted hydroxypropyl cellulose, magnesium stearate, microcrystalline cellulose, and FD&C Blue No. 2 Aluminum Lake as colorant.
6PATIENT COUNSELING INFORMATION
As a part of patient counseling, healthcare providers must review the SAMSCA Medication Guide with every patient
7MEDICATION GUIDE
SAMSCA
Read the Medication Guide that comes with SAMSCA before you take it and each time you get a new prescription. There may be new information. This Medication Guide does not take the place of talking to your healthcare provider about your medical condition or your treatment. Share this important information with members of your household.
What is the most important information I should know about SAMSCA?
1)
- trouble speaking
- swallowing trouble or feeling like food or liquid gets stuck while swallowing
- drowsiness
- confusion
- mood changes
- trouble controlling body movement (involuntary movement) and weakness in muscles of the arms and legs
- seizures
You or a family member should tell your healthcare provider right away if you have any of these symptoms even if they begin later in treatment. Also tell your healthcare provider about any other new symptoms while taking SAMSCA.
You may be more at risk for ODS if you have:
- liver disease
- not eaten enough for a long period of time (malnourished)
- very low sodium level in your blood
- been drinking large amounts of alcohol for a long period of time (chronic alcoholism)
To lessen your risk of ODS while taking SAMSCA:
- Treatment with SAMSCA should be started and re-started only in a hospital, where the sodium levels in your blood can be checked closely.
- Do not take SAMSCA if you cannot tell if you are thirsty.
- To prevent losing too much body water (dehydration), have water available to drink at all times while taking SAMSCA. Unless your healthcare provider tells you otherwise, drink when you are thirsty.
- If your healthcare provider tells you to keep taking SAMSCA after you leave a hospital, it is important that you do not stop and re-start SAMSCA on your own. You may need to go back to a hospital to re-start SAMSCA. Talk to your healthcare provider right away if you stop taking SAMSCA for any reason.
- It is important to stay under the care of your healthcare provider while taking SAMSCA and follow their instructions.
2)
- Loss of appetite, nausea, vomiting
- Fever, feeling unwell, unusual tiredness
- Itching
- Yellowing of the skin or the whites of the eyes (jaundice)
- Unusual darkening of the urine
- Right upper stomach area pain or discomfort
3)
What is SAMSCA?
SAMSCA is a prescription medicine used to help increase low sodium levels in the blood, in adults with conditions such as heart failure, and certain hormone imbalances. SAMSCA helps raise salt levels in your blood by removing extra body water as urine.
It is not known if SAMSCA is safe or works in children.
Who should not take SAMSCA?
Do not take SAMSCA if:
- you are allergic to tolvaptan or any of the ingredients in SAMSCA. See the end of this Medication Guide for a complete list of ingredients in SAMSCA.
- the sodium level in your blood must be increased right away.
- you cannot replace fluids by drinking or you cannot feel if you are thirsty.
- you are dizzy, faint, or your kidneys are not working normally because you have lost too much body fluid.
- you take certain medicines. These medicines could cause you to have too much SAMSCA in your blood:
- your body is not able to make urine. SAMSCA will not help your condition.
What should I tell my healthcare provider before taking SAMSCA?
Tell your healthcare provider about all your medical conditions, including if you:
- have kidney problems and your body cannot make urine.
- have liver problems
- cannot feel if you are thirsty. See "
- had an allergic reaction in the past to tolvaptan or to any of the other ingredients of this medicine. See the end of this Medication Guide for a list of the ingredients in SAMSCA.
- are pregnant or plan to become pregnant. It is not known if SAMSCA will harm your unborn baby.
- are breast-feeding. It is not known if SAMSCA passes into your breast milk. You and your healthcare provider should decide if you will take SAMSCA or breast-feed. You should not do both.
- are taking desmopressin (dDAVP).
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
Using SAMSCA with certain medicines could cause you to have too much SAMSCA in your blood. See "
SAMSCA may affect the way other medicines work and other medicines may affect how SAMSCA works.
Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take SAMSCA?
- See "
- Take SAMSCA exactly as prescribed by your healthcare provider.
- Take SAMSCA one time each day.
- You can take SAMSCA with or without food.
- Do not drink grapefruit juice during treatment with SAMSCA. This could cause you to have too much SAMSCA in your blood.
- Certain medicines or illnesses may keep you from drinking fluids or may cause you to lose too much body fluid, such as vomiting or diarrhea. If you have these problems, call your healthcare provider right away.
- Do not miss or skip doses of SAMSCA. If you miss a dose, take it as soon as you remember. If it is near the time of the next dose, skip the missed dose. Just take the next dose at your regular time. Do not take 2 doses at the same time.
- If you take too much SAMSCA, call your healthcare provider right away. If you take an overdose of SAMSCA, you may need to go to a hospital.
- If your healthcare provider tells you to stop taking SAMSCA, follow their instructions about limiting the amount of fluid you should drink.
What are the possible side effects of SAMSCA?
SAMSCA can cause serious side effects including:
- See "
- Loss of too much body fluid (dehydration). Tell your healthcare provider if you:
- have vomiting or diarrhea and cannot drink normally.
- feel dizzy or faint. These may be symptoms that you have lost too much body fluid.
Call your healthcare provider right away if you have any of these symptoms.
The most common side effects of SAMSCA are:
- thirst
- dry mouth
- weakness
- constipation
- making large amounts of urine and urinating often
- increased blood sugar levels
These are not all the possible side effects of SAMSCA. Talk to your healthcare provider about any side effect that bothers you or that does not go away while taking SAMSCA.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store SAMSCA?
Store SAMSCA between 59°F to 86°F (15°C to 30°C).
Keep SAMSCA and all medicines out of the reach of children.
General Information about SAMSCA
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use SAMSCA for a condition for which it was not prescribed. Do not give SAMSCA to other people, even if they have the same symptoms you have. It may harm them.
This Medication Guide summarizes the most important information about SAMSCA. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about SAMSCA that is written for healthcare professionals. For more information about SAMSCA, call 1-877-726-7220 or go to www.samsca.com.
What are the ingredients in SAMSCA?
Active ingredient: tolvaptan.
Inactive ingredients: corn starch, hydroxypropyl cellulose, lactose monohydrate, low-substituted hydroxypropyl cellulose, magnesium stearate, microcrystalline cellulose, and FD&C Blue No. 2 Aluminum Lake as colorant.
Manufactured by Otsuka Pharmaceutical Co., Ltd., Tokyo, 101-8535 Japan
Distributed and marketed by Otsuka America Pharmaceutical, Inc., Rockville, MD 20850
SAMSCA is a registered trademark of Otsuka Pharmaceutical Co., Ltd., Tokyo, 101-8535 Japan
Rev. 4/2021
This Medication Guide has been approved by the U.S. Food and Drug Administration.
©2021 Otsuka Pharmaceutical Co., Ltd.
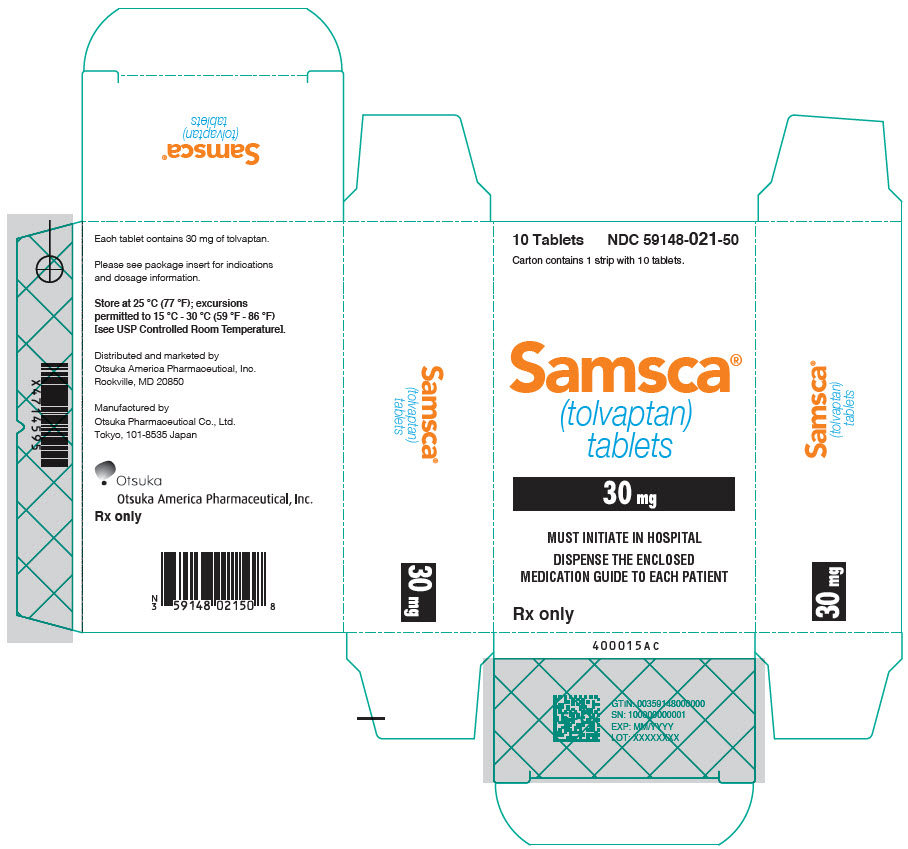
8PRINCIPAL DISPLAY PANEL - 15 mg Tablet Blister Pack Carton
10 Tablets
Carton contains 1 strip with 10 tablets.
Samsca
15 mg
MUST INITIATE IN HOSPITAL
DISPENSE THE ENCLOSED
Rx only
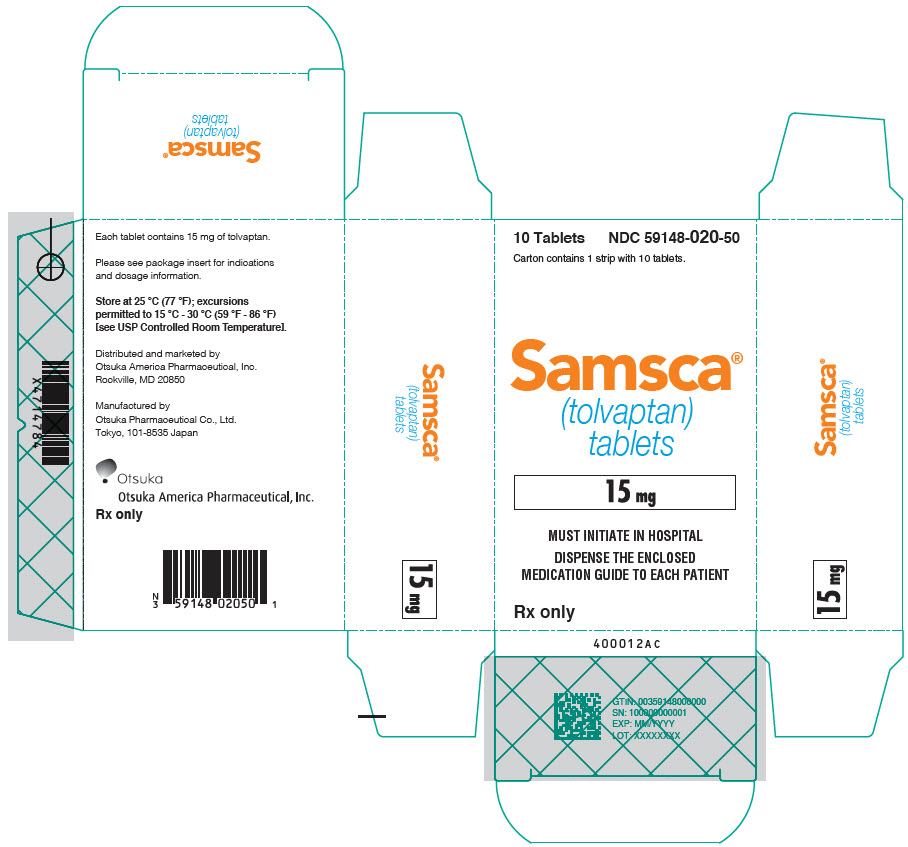
9PRINCIPAL DISPLAY PANEL - 30 mg Tablet Blister Pack Carton
10 Tablets
Carton contains 1 strip with 10 tablets.
Samsca
30 mg
MUST INITIATE IN HOSPITAL
DISPENSE THE ENCLOSED
Rx only