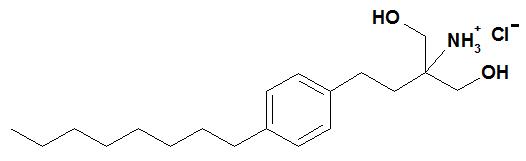
Fingolimod
What is Gilenya (Fingolimod)?
Approved To Treat
Related Clinical Trials
Summary: The purpose of this study is to evaluate the effectiveness, safety, tolerability, drug levels and drug effects of ozanimod compared to fingolimod in children and adolescents with relapsing remitting multiple sclerosis (RRMS).
Summary: The aim of this study is to explore the efficacy and safety of Fingoland in the treatment of type 2 diabetes. A total of 40 patients were randomly divided into two groups. One group was treated with fingolimod, another group was given guideline based treatment. The changes of islet function in patients with glycosylated hemoglobin, insulin and C-peptide were observed .
Summary: This is a single-institution, open-labeled study using fingolimod (FTY720/Gilenya) in patients with non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) who have progressed on chemo-immunotherapy. The study design will be a 6 patient safety lead-in with 2 cohorts of patients for efficacy analysis where fingolimod 0.5 mg will be taken orally once daily.
Related Latest Advances
Brand Information
- 0.25 mg hard capsules with an ivory opaque body and cap, with black radial imprint “FTY 0.25 mg” on the cap and a black radial band on the capsule body.
- 0.5 mg hard capsules with a white opaque body and bright yellow cap imprinted with “FTY 0.5 mg” on the cap and 2 radial bands imprinted on the capsule body with yellow ink.
- in the last 6 months experienced myocardial infarction, unstable angina, stroke, transient ischemic attack (TIA), decompensated heart failure requiring hospitalization or Class III/IV heart failure
- a history or presence of Mobitz Type II second-degree or third-degree AV block or sick sinus syndrome, unless patient has a functioning pacemaker
- a baseline QTc interval ≥ 500 msec
- cardiac arrhythmias requiring anti-arrhythmic treatment with Class Ia or Class III anti-arrhythmic drugs
- had a hypersensitivity reaction to fingolimod or any of the excipients in GILENYA. Observed reactions include rash, urticaria and angioedema upon treatment initiation
- Bradyarrhythmia and Atrioventricular Blocks
- Infections
- Progressive Multifocal Leukoencephalopathy
- Macular Edema
- Liver Injury
- Posterior Reversible Encephalopathy Syndrome
- Respiratory Effects
- Fetal Risk
- Severe Increase in Disability After Stopping GILENYA
- Tumefactive Multiple Sclerosis
- Increased Blood Pressure
- Malignancies
- Immune System Effects Following GILENYA Discontinuation
- Hypersensitivity Reactions
- Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females to inform their healthcare provider of a known or suspected pregnancy
- Advise female patients of reproductive potential to use effective contraception during treatment with GILENYA and for 2 months after the final dose