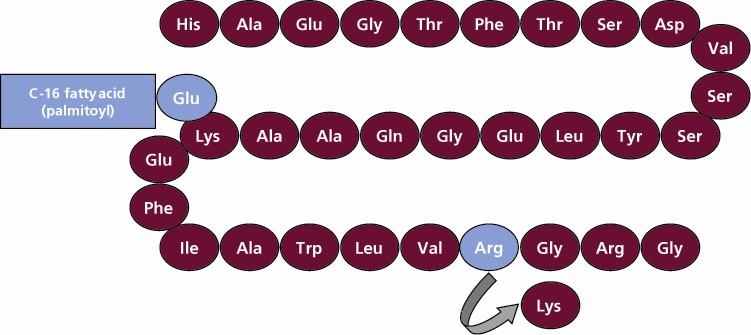
Liraglutide
What is Victoza (Liraglutide)?
Related Clinical Trials
Summary: The investigators recently demonstrated that blockade of Glucagon-Like Peptide-1's (GLP-1) receptor (GLP1R) results in changes in islet function without changes in circulating GLP-1. These effects are more pronounced in people with early type 2 diabetes (T2DM) in keeping with increased expression of PC-1/3 and GLP-1 that is observed in diabetic islets. However, its regulation is at present unknown...
Summary: This study is for people who have multiple sclerosis, acute leukemia (in remission), or long-COVID and a Body Mass Index over 27 and may struggle with cognitive issues such as remembering information, concentrating, or making decisions that affect everyday life. By doing this study, researchers hope to learn how liraglutide (Saxenda®), a weight loss drug, affects levels of a certain disease marker...
Summary: This study is open to adults with obesity. People with a body mass index (BMI) in the range from 30 to 40 kg/m2 and a body weight of 70 to 150 kg can participate in the study. The purpose of this study is to find out whether treatment with a medicine called BI 456906 changes the occupancy of receptors in the liver and in the pancreas. These receptors are involved in appetite and weight regulation....
Related Latest Advances
Brand Information
- Liraglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures in both genders of rats and mice. It is unknown whether VICTOZA causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans, as the human relevance of liraglutide-induced rodent thyroid C-cell tumors has not been determined
- VICTOZA is contraindicated in patients with a personal or family history of MTC and in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk for MTC with the use of VICTOZA and inform them of symptoms of thyroid tumors (e.g., a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with VICTOZA
- as an adjunct to diet and exercise to improve glycemic control in patients 10 years and older with type 2
- diabetes mellitus,
- to reduce the risk of major adverse cardiovascular events (cardiovascular death, non-fatal myocardial
- Medullary Thyroid Carcinoma
- Hypersensitivity
- Risk of Thyroid C-cell Tumors
- Pancreatitis
- Hypoglycemia
- Renal Impairment
- Hypersensitivity Reactions
- Acute Gallbladder Disease
- Medullary thyroid carcinoma
- Dehydration resulting from nausea, vomiting and diarrhea.
- Increased serum creatinine, acute renal failure or worsening of chronic renal failure, sometimes requiring hemodialysis.
- Angioedema and anaphylactic reactions.
- Allergic reactions: rash and pruritus
- Skin and subcutaneous tissue disorder: cutaneous amyloidosis
- Acute pancreatitis, hemorrhagic and necrotizing pancreatitis sometimes resulting in death
- Hepatobiliary disorders: hyperbilirubinemia, elevations of liver enzymes, cholestasis, hepatitis, cholecystitis, cholelithiasis requiring cholecystectomy
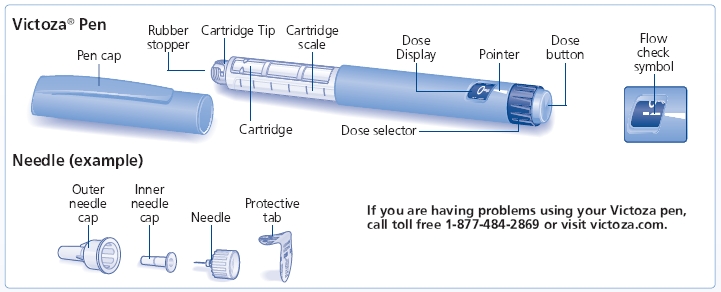
- Δ Always use a new needle for each injection to prevent contamination.
- Δ Always remove the needle after each injection, and store your pen without the needle
- attached. This reduces the risk of contamination, infection, leakage of liraglutide,
- blocked needles and inaccurate dosing.
- Δ Keep your Victoza pen and all medicines out of the reach of children.
- Δ If you drop your Victoza pen, repeat “First Time Use For Each New Pen” (steps A
- through D).
- Δ Be careful not to bend or damage the needle.
- Δ Do not use the cartridge scale to measure how much Victoza to inject.
- Δ Be careful when handling used needles to avoid needle stick injuries.
- Δ You can use your Victoza pen for up to 30 days after you use it the first time.
- Take your new Victoza pen out of the refrigerator.
- Wash hands with soap and water before use.
- Check pen label before each use to make sure it is your Victoza pen.
- Pull off pen cap (See Figure A).
- Check Victoza in the cartridge. The liquid should be clear, colorless and free of particles. If not, do not use.
- Wipe the rubber stopper with an alcohol swab.
- Remove protective tab from outer needle cap (See Figure B).
- Push outer needle cap containing the needle straight onto the pen, then screw needle on until secure.
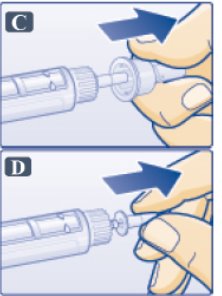
- Pull off outer needle cap (See Figure C). Do not throw away
- Pull off inner needle cap and throw away (See Figure D). A small drop of liquid may appear. This is normal.
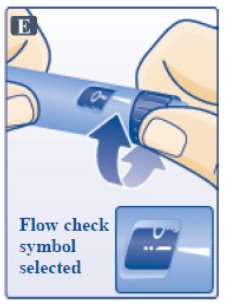
- Turn dose selector until flow check symbol (--) lines up with pointer (See Figure E). The flow check symbol does not administer the dose as prescribed by your healthcare provider.
- To select the dose prescribed by your healthcare provider, continue to Step G under “Routine Use”.
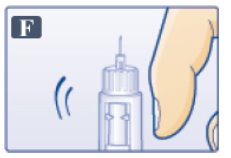
- Hold pen with needle pointing up.
- Tap cartridge gently with your finger a few times to bring any air bubbles to the top of the cartridge (See Figure F).
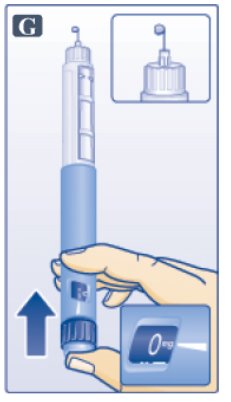
- Keep needle pointing up and press dose button until 0 mg lines up with pointer (See Figure G). Repeat steps C and D, up to 6 times, until a drop of Victoza appears at the needle tip.
- Take your Victoza pen from where it is stored.
- Wash hands with soap and water before use.
- Check pen label before each use to make sure it is your Victoza pen.
- Pull off pen cap (See Figure H).
- Check Victoza in the cartridge. The liquid should be clear, colorless and free of particles. If not, do not use.
- Wipe the rubber stopper with an alcohol swab.
- Remove protective tab from outer needle cap.
- Push outer needle cap containing the needle straight onto the pen, then screw needle on until secure (See Figure I).
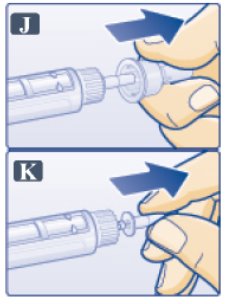
- Pull off outer needle cap. Do not throw away (See Figure J).
- Pull off inner needle cap and throw away (See Figure K). A small drop of liquid may appear. This is normal.
- Victoza pen can give a dose of 0.6 mg (starting dose), 1.2 mg or 1.8 mg. Be sure that you know the dose of Victoza that is prescribed for you.
- Turn the dose selector until your needed dose lines up with the pointer (0.6 mg, 1.2 mg or 1.8 mg) (See Figure L).
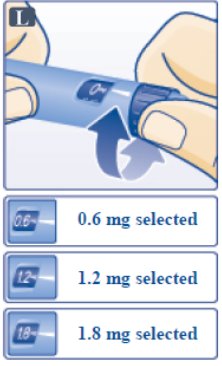
- You will hear a "click" every time you turn the dose selector.
- If you select a wrong dose, change it by turning the dose selector backwards or forwards until the correct dose lines up with the pointer. Be careful not to press the dose button when turning the dose selector. This may cause Victoza to come out.
- Insert needle into your skin in the stomach (abdomen), thigh or upper arm. Use the injection technique shown to you by your healthcare provider.
- Press down on the center of the dose button to inject until 0 mg lines up with the pointer (See Figure M).
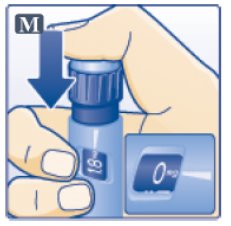
- Be careful not to touch the dose display with your other fingers. This may block the injection.
- Keep the dose button pressed down and make sure that you keep the needle under the skin for a full count of 6 seconds to make sure the full dose is injected. Keep your thumb on the injection button until you remove the needle from your skin (See Figure N).
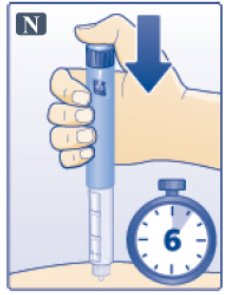
- Change (rotate) your injection sites within the area you choose for each dose.
- You may see a drop of Victoza at the needle tip. This is normal and it does not affect the dose you just received. If blood appears after you take the needle out of your skin, apply light pressure, but
- Carefully put the outer needle cap over the needle (See Figure P). Unscrew the needle.
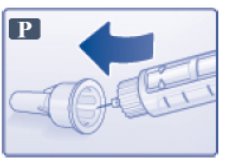
- Safely remove the needle from your Victoza pen after each use.
- Put your used VICTOZA pen and needles in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and pens in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
- upright and stable during use
- leak-resistant
- properly labeled to warn of hazardous waste inside the container
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. Do not reuse or share your needles with other people. For more information about the safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at:
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
- After removing the needle, put the pen cap on your Victoza pen and store your Victoza pen without the needle attached (See Figure Q).
- Do not try to refill your Victoza pen - it is prefilled and is disposable.
- Do not try to repair your pen or pull it apart.
- Keep your Victoza pen away from dust, dirt and liquids.
- If cleaning is needed, wipe the outside of the pen with a clean, damp cloth.
- Store your new, unused Victoza pen in the refrigerator at 36ºF to 46ºF (2ºC to 8ºC).
- If Victoza is stored outside of refrigeration (by mistake) prior to first use, it should be used or thrown away within 30 days.
- Do not freeze Victoza or use Victoza if it has been frozen. Do not store Victoza near the refrigerator cooling element.
- Use a Victoza pen for only 30 days. Throw away a used Victoza pen 30 days after you start using it, even if some medicine is left in the pen.
- Store your Victoza pen at 59ºF to 86ºF (15ºC to 30ºC), or in a refrigerator at 36ºF to 46ºF (2°C to 8°C).
- When carrying the pen away from home, store the pen at a temperature between 59ºF to 86ºF (15ºC to 30ºC).
- If Victoza has been exposed to temperatures above 86ºF (30°C), it should be thrown away.
- Protect your Victoza pen from heat and sunlight.
- Keep the pen cap on when your Victoza pen is not in use.
- Always remove the needle after each injection and store your pen without the needle attached. This reduces the risk of contamination, infection, leakage and inaccurate dosing.





