Generic Name
Nepafenac
Brand Names
Ilevro, Nevanac
FDA approval date: February 23, 2024
Classification: Nonsteroidal Anti-inflammatory Drug
Form: Suspension
What is Ilevro (Nepafenac)?
ILEVRO ® 0.3% is indicated for the treatment of pain and inflammation associated with cataract surgery. ILEVRO ® 0.3% is a nonsteroidal, anti-inflammatory prodrug indicated for the treatment of pain and inflammation associated with cataract surgery .
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
Comparing Efficacy of Bromfenac 0.09%, Nepafenac 0.3% and Diclofenac 0.1% in Patients After Cataract Surgery
Summary: The goal of this clinical trial is to learn which of the drugs bromfenac 0.09%, nepafenac 0.3% and diclofenac 0.1% has better efficacy in treatment and prevention of cystoid macular oedema after cataract surgery. The main questions it aims to answer are: Which of the drugs has better efficacy in macular oedema prevention? What medical problems do participants have when taking different drugs? Rese...
A Randomized Clinical Two-arm Trial Comparing Inflammation and Cystoid Macular Edema for the Medication Regimens Postoperative Topical NSAIDs and Steroids to Only Postoperative Topical Steroids in Patients Undergoing Corneal Endothelial Transplantations (DMEC)
Summary: The DMEC trial is a randomized clinical two-arm trial comparing inflammation and cystoid macular edema for the medication regimens postoperative topical NSAIDs and steroids to only postoperative topical steroids in patients undergoing corneal endothelial transplantations (DSAEK or DMEK).
Related Latest Advances
Brand Information
ILEVRO (nepafenac)
1INDICATIONS AND USAGE
ILEVRO
2DOSAGE FORMS AND STRENGTHS
Sterile ophthalmic suspension 0.3%: 1.7 mL in a 4 mL bottle and 3 mL in a 4 mL bottle.
3CONTRAINDICATIONS
ILEVRO
4ADVERSE REACTIONS
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to the rates in the clinical studies of another drug and may not reflect the rates observed in practice.
4.1Serious and Otherwise Important Adverse Reactions
The following adverse reactions are discussed in greater detail in other sections of labeling.
- Increased Bleeding Time
- Delayed Healing
- Corneal Effects
4.2Ocular Adverse Reactions
The most frequently reported ocular adverse reactions following cataract surgery were capsular opacity, decreased visual acuity, foreign body sensation, increased intraocular pressure (IOP), and sticky sensation. These reactions occurred in approximately 5% to 10% of patients.
Other ocular adverse reactions occurring at an incidence of approximately 1% to 5% included conjunctival edema, corneal edema, dry eye, lid margin crusting, ocular discomfort, ocular hyperemia, ocular pain, ocular pruritus, photophobia, tearing, and vitreous detachment.
Some of these reactions may be the consequence of the cataract surgical procedure.
4.3Non-Ocular Adverse Reactions
Non-ocular adverse reactions reported at an incidence of 1% to 4% included headache, hypertension, nausea/vomiting, and sinusitis.
5DESCRIPTION
ILEVRO
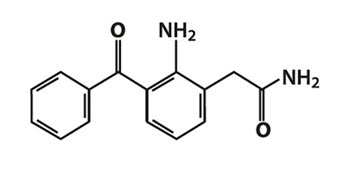
Nepafenac is a yellow crystalline powder. The molecular weight of nepafenac is 254.28 g/mol. ILEVRO
The osmolality of ILEVRO
Each mL of ILEVRO
6CLINICAL STUDIES
In two double masked, randomized clinical trials in which patients were dosed daily beginning one day prior to cataract surgery, continued on the day of surgery and for the first two weeks of the postoperative period, ILEVRO
Treatment effect over vehicle for resolution of ocular pain occurred as early as Day 1 post-surgery. Treatment effect over vehicle for resolution of inflammation was significantly better than vehicle in both studies at Day 7 and Day 14 post-surgery.
7HOW SUPPLIED/STORAGE AND HANDLING
ILEVRO
3 mL in 4 mL bottle NDC 82667-400-03
Storage:Store at 2°C to 25°C (36°F to 77°F).
Protect from light.
8PATIENT COUNSELING INFORMATION
Slow or Delayed Healing
Inform the patient of the possibility that slow or delayed healing may occur while using NSAIDs
Avoiding Contamination of the Product
Instruct the patient to avoid allowing the tip of the dispensing container to contact the eye or surrounding structures because this could cause the tip to become contaminated by common bacteria known to cause ocular infections. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions.
Use of the same bottle for both eyes is not recommended with topical eye drops that are used in association with surgery.
Contact Lens Wear
ILEVRO
Intercurrent Ocular Conditions
Advise the patient that if they develop an intercurrent ocular condition (e.g., trauma, or infection) or have ocular surgery, they should immediately seek their physician’s advice concerning the continued use of the multi-dose container
Concomitant Topical Ocular Therapy
If more than one topical ophthalmic medication is being used, the medicines must be administered at least 5 minutes apart
Shake Well Before Use
Patients should be instructed to shake well before each use
9PRINCIPAL DISPLAY PANEL
NDC 82667-400-03
STERILE
ILEVRO
(nepafenac ophthalmic suspension) 0.3%
3 mL
Harrow Eye, LLC
HARROW
