Bimatoprost
What is Durysta (Bimatoprost)?
Approved To Treat
Related Clinical Trials
Summary: This is a multicenter, open-label, dose escalation (Cohort 1) to masked, randomized, parallel-groups (Cohort 2) and (Cohort 3) study to evaluate the safety and efficacy of AGN-193408 SR in participants with open-angle glaucoma or ocular hypertension
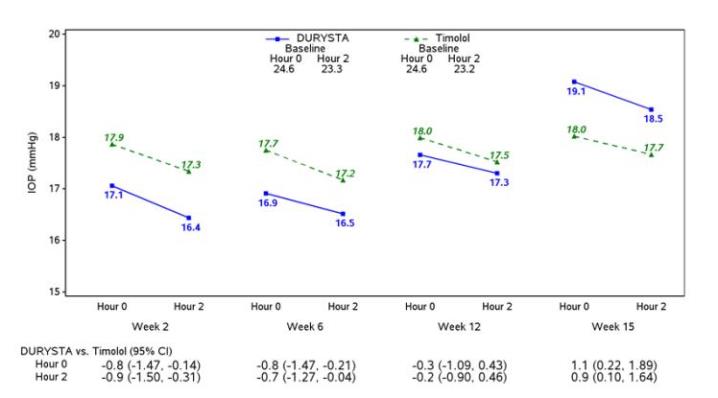
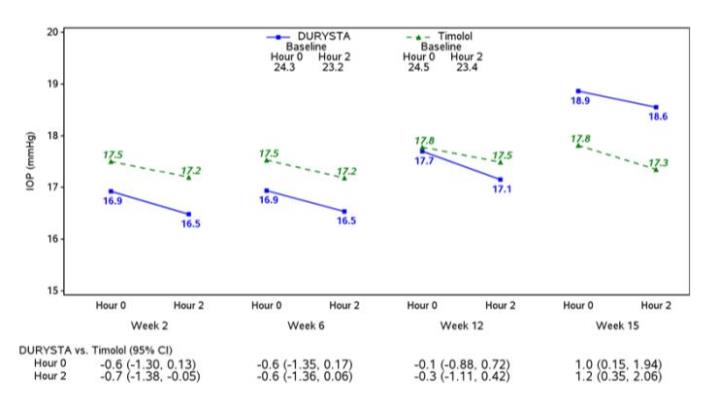
Summary: This trial is a randomized study to evaluate the Bimatoprost Implant System used in combination with the SpyGlass IOL to Timolol Ophthalmic Solution in participants with mild to moderate open-angle glaucoma or ocular hypertension undergoing cataract surgery.
Summary: This trial is a randomized study to evaluate the Bimatoprost Implant System used in combination with the SpyGlass IOL to Timolol Ophthalmic Solution in participants with mild to moderate open-angle glaucoma or ocular hypertension undergoing cataract surgery.
Related Latest Advances
Brand Information
- Implant migration
- Hypersensitivity
- Corneal adverse reactions
- Macular edema
- Intraocular inflammation
- Pigmentation
- Endophthalmitis
![The chemical name for bimatoprost is (Z)-7-[(1R,2R,3R,5S)-3,5-dihydroxy-2-[(1E,3S)-3-hydroxy-5-phenyl-1-pentenyl]cyclopentyl]-N-ethyl-5-heptenamide, and its molecular weight is 415.57. Its molecular formula is C25H37NO4.](https://dailymed.nlm.nih.gov/dailymed/image.cfm?name=durysta-03.jpg&setid=3f59da84-0bcc-4c84-b3e2-e215681ef341)