Brand Name
Xenpozyme
Generic Name
Olipudase Alfa-Rpcp
View Brand Information FDA approval date: August 29, 2022
Form: Injection
What is Xenpozyme (Olipudase Alfa-Rpcp)?
XENPOZYME is indicated for treatment of non–central nervous system manifestations of acid sphingomyelinase deficiency in adult and pediatric patients. XENPOZYME is a hydrolytic lysosomal sphingomyelin-specific enzyme indicated for treatment of non–central nervous system manifestations of acid sphingomyelinase deficiency in adult and pediatric patients.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
XENPOZYME (olipudase alfa-rpcp)
WARNING: HYPERSENSITIVITY REACTIONS INCLUDING ANAPHYLAXIS
Patients treated with XENPOZYME have experienced life-threatening hypersensitivity reactions, including anaphylaxis. Appropriate medical monitoring and support measures, including cardiopulmonary resuscitation equipment, should be readily available during XENPOZYME administration. If a severe hypersensitivity reaction (e.g., anaphylaxis) occurs, discontinue XENPOZYME immediately and initiate appropriate medical treatment. In patients with severe hypersensitivity reactions, a desensitization procedure to XENPOZYME may be considered
1INDICATIONS AND USAGE
XENPOZYME is indicated for treatment of non–central nervous system manifestations of acid sphingomyelinase deficiency (ASMD) in adult and pediatric patients.
2DOSAGE FORMS AND STRENGTHS
For injection: 4 mg or 20 mg of olipudase alfa-rpcp as a sterile, white to off white lyophilized powder in a single-dose vial for reconstitution.
3CONTRAINDICATIONS
None.
4ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Hypersensitivity Reactions Including Anaphylaxis
- Infusion-Associated Reactions (IARs)
- Elevated Transaminase Levels
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The pooled safety analysis from 3 clinical trials included a total of 38 XENPOZYME-treated patients (30 adult and 8 pediatric patients) with age range from 1.5 to 59 years old receiving intravenous doses up to 3 mg/kg every 2 weeks
Serious adverse reactions of anaphylactic reaction were reported in 2 (25%) XENPOZYME-treated pediatric patients.
Most frequently reported adverse drug reactions in adults (incidence ≥10%) were headache, cough, diarrhea, hypotension, and ocular hyperemia.
Most frequently reported adverse drug reactions in pediatric patients (incidence ≥20%) were pyrexia, cough, diarrhea, rhinitis, abdominal pain, vomiting, headache, urticaria, nausea, rash, arthralgia, pruritus, fatigue, and pharyngitis.
5OVERDOSAGE
Cases of overdosage with XENPOZYME have been reported in pediatric patients during dose escalation. Some patients experienced serious adverse reactions including death within 24 hours of initial dose
There is no known specific antidote for XENPOZYME overdosage. In the event of overdosage, immediately stop the infusion, and monitor the patient closely in a hospital setting for the development of hypersensitivity reactions and IARs including acute phase reactions. For the management of adverse reactions,
6DESCRIPTION
Olipudase alfa-rpcp is a hydrolytic lysosomal sphingomyelin-specific enzyme consisting of 570 amino acids produced in a Chinese hamster ovary cell line by recombinant DNA technology. The molecular weight of olipudase alfa-rpcp is approximately 76 kDa.
XENPOZYME (olipudase alfa-rpcp) for injection is supplied as a sterile, preservative-free, white to off-white lyophilized powder for reconstitution and dilution to be administered via intravenous infusion. XENPOZYME is supplied in single-dose vials.
Each 4 mg vial contains 4 mg olipudase alfa-rpcp, dibasic sodium phosphate (0.89 mg), methionine (14.92 mg), monobasic sodium phosphate (1.63 mg), and sucrose (50 mg). After reconstitution with 1.1 mL of Sterile Water for Injection, USP, the final concentration is 4 mg/mL
Each 20 mg vial contains 20 mg olipudase alfa-rpcp, dibasic sodium phosphate (4.47 mg), methionine (74.6 mg), monobasic sodium phosphate (8.17 mg), and sucrose (250 mg). After reconstitution with 5.1 mL of Sterile Water for Injection, USP, the final concentration is 4 mg/mL
The pH is 6.5 after reconstitution.
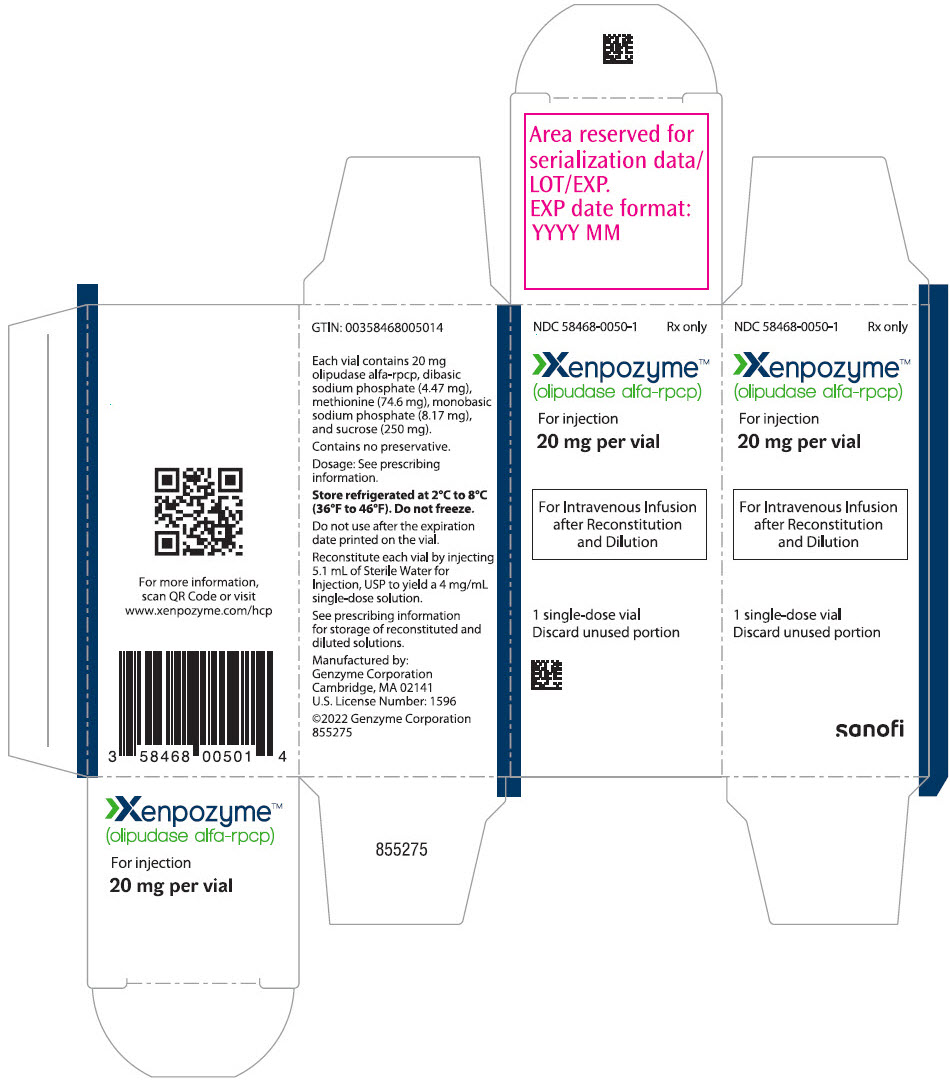
7PRINCIPAL DISPLAY PANEL - 20 mg Vial Carton
NDC 58468-0050-1
Xenpozyme™
For injection
20 mg per vial
For Intravenous Infusion
1 single-dose vial
8PRINCIPAL DISPLAY PANEL - 4 mg Vial Carton
NDC 58468-0051-1
Xenpozyme™
For injection
4 mg per vial
For Intravenous Infusion
1 single-dose vial
