Generic Name
CycloSPORINE
Brand Names
CEQUA, Restasis MultiDose, Sandimmune, Gengraf, Neoral, Restasis, VEVYE, Verkazia
FDA approval date: November 14, 1983
Classification: Calcineurin Inhibitor Immunosuppressant
Form: Injection, Emulsion, Capsule, Solution
What is CEQUA (CycloSPORINE)?
Kidney, Liver, and Heart Transplantation Cyclosporine capsules is indicated for the prophylaxis of organ rejection in kidney, liver, and heart allogeneic transplants. Cyclosporine capsules has been used in combination with azathioprine and corticosteroids. Rheumatoid Arthritis Cyclosporine capsules is indicated for the treatment of patients with severe active, rheumatoid arthritis where the disease has not adequately responded to methotrexate. Cyclosporine capsules can be used in combination with methotrexate in rheumatoid arthritis patients who do not respond adequately to methotrexate alone. Psoriasis Cyclosporine capsules is indicated for the treatment of adult, nonimmunocompromised patients with severe , recalcitrant, plaque psoriasis who have failed to respond to at least one systemic therapy or in patients for whom other systemic therapies are contraindicated or cannot be tolerated. While rebound rarely occurs, most patients will experience relapse with cyclosporine capsules as with other therapies upon cessation of treatment.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
CEQUA (cyclosporine)
1INDICATIONS AND USAGE
CEQUA ophthalmic solution is a calcineurin inhibitor immunosuppressant indicated to increase tear production in patients with keratoconjunctivitis sicca (dry eye). (
2DOSAGE AND ADMINISTRATION
Instill one drop of CEQUA twice daily (approximately 12 hours apart) into each eye. CEQUA can be used concomitantly with artificial tears, allowing a 15 minute interval between products. Discard the vial immediately after using in both eyes. (
3DOSAGE FORMS AND STRENGTHS
Ophthalmic solution containing cyclosporine 0.9 mg/mL (
4CONTRAINDICATIONS
None. (
5DESCRIPTION
CEQUA (cyclosporine ophthalmic solution) 0.09% contains a topical calcineurin inhibitor immunosuppressant. Cyclosporine’s chemical name is Cyclo[[(E)-(2S,3R,4R)-3-hydroxy-4-methyl-2-(methylamino)-6-octenoyl]-L-2-aminobutyryl-N-methylglycyl-N-methyl-L-leucyl-L-valyl-N-methyl-L-leucyl-L-alanyl-D-alanyl-N-methyl-L-leucyl-N-methyl-L-leucyl-N-methyl-L-valyl] and it has the following structure:
Structural Formula
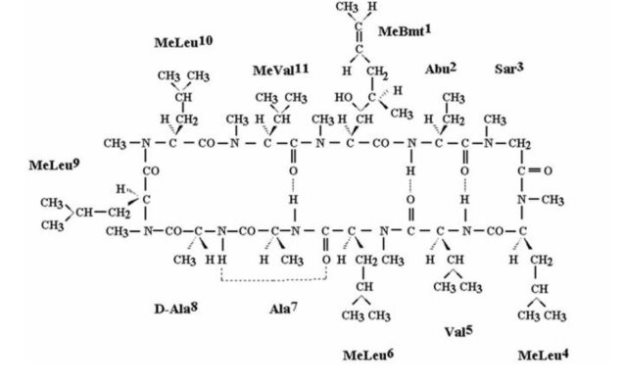
Formula: C
Cyclosporine is a white powder that is insoluble in water. CEQUA is supplied as a sterile, clear, colorless ophthalmic solution for topical ophthalmic use. It has an osmolality of 160 to 190 mOsmol/kg and a pH of 6.5-7.2. Each mL of CEQUA contains:
- Active: cyclosporine 0.09%
- Inactives: Polyoxyl 40 Hydrogenated Castor Oil, Octoxynol-40, polyvinylpyrrolidone, sodium phosphate monobasic dihydrate, sodium phosphate dibasic anhydrous, sodium chloride, water for injection, and sodium hydroxide or hydrochloric acid to adjust pH.
6CLINICAL STUDIES
Two multicenter, randomized, adequate and well-controlled clinical studies treated 1,048 patients with keratoconjunctivitis sicca (NCT # 02254265 and NCT # 02688556). In both studies, compared to vehicle at Day 84, there was a statistically significant (p<0.01) higher percentage of eyes with increases of ≥ 10 mm from baseline in Schirmer wetting. This effect was seen in approximately 17% of CEQUA-treated patients versus approximately 9% of vehicle-treated patients.
7HOW SUPPLIED/STORAGE AND HANDLING
CEQUA ophthalmic solution is packaged in sterile, preservative-free, single-use vials. Each vial
contains 0.25 mL fill in a 0.9 mL LDPE vial; 10 vials (2 cards of 5 vials) are packaged in a
polyfoil aluminum pouch; 6 pouches are packaged in a box. The entire contents of each box of
60 vials must be dispensed intact.
60 Single-Use Vials 0.25 mL each - NDC 47335-506-96
Storage: Store at 20°C to 25°C (68°F to 77°F). Store single-use vials in the original foil pouch.
8PATIENT COUNSELING INFORMATION
Handling the Vial
Advise patients to not allow the tip of the vial to touch the eye or any surface, as this may
contaminate the solution. Advise patients also not to touch the vial tip to their eye to avoid the
potential for injury to the eye
Use with Contact Lenses
CEQUA should not be administered while wearing contact lenses. Patients with decreased tear
production typically should not wear contact lenses. Advise patients that if contact lenses are
worn, they should be removed prior to the administration of the solution. Lenses may be
reinserted 15 minutes following administration of CEQUA ophthalmic solution
Administration
Advise patients that the solution from one individual single-use vial is to be used immediately
after opening for administration to one or both eyes, and the remaining contents should be
discarded immediately after administration.
Rx Only
Manufactured for: Sun Pharmaceutical Industries Limited
By: Laboratoire Unither
1 rue de l’Arquerie
50200 Coutances
France
Cyclosporine (active ingred.) Product of Czech Republic.
Product of France
Distributed by:
Sun Pharmaceutical Industries, Inc.
Cranbury, NJ 08512
Copyright 2022, Sun Pharmaceutical Industries Limited
All rights reserved
07/2022
uspi-CEQUA-sol-00004