Intuniv
What is Intuniv (Guanfacine)?
Approved To Treat
Related Clinical Trials
Summary: The investigators assess whether guanfacine extended release (GXR; 3mg/d) compared with placebo (PBO) will attenuate drinking and drinking-related factors in N=200 men and women with Alcohol Use Disorder (AUD) across 12-weeks.
Summary: The US is currently going through an opioid crisis, and while Medication Assisted Treatments such as buprenorphine (BUP) have proved highly effective at stabilizing the neurobiology underlying acute withdrawal, they have been less effective at preventing longer-term relapse and adherence. This may be due to the fact that they do not fully engage the neural processes sub-serving the emotional contr...
Summary: A randomized controlled trial to assess the efficacy of extended-release guanfacine to reduce cannabis use frequency in young people with cannabis use disorder following a period of monitored abstinence.
Related Latest Advances
Brand Information
- Hypotension, bradycardia, and syncope
- Sedation and somnolence
- Cardiac conduction abnormalities
- Rebound Hypertension [see
- Five short-term, placebo-controlled monotherapy trials (Studies 1, 2, 4, 5, and 6).
- One short-term, placebo-controlled adjunctive trial with psychostimulants (Study 3).
- One long-term, placebo-controlled monotherapy maintenance trial (Study 7).
- have heart problems or a low heart rate
- have fainted
- have low or high blood pressure
- have liver or kidney problems
- have any other medical conditions
- are pregnant or plan to become pregnant. It is not known if INTUNIV will harm your unborn baby. Talk to your doctor if you are pregnant or plan to become pregnant.
- are breastfeeding or plan to breastfeed. It is not known if INTUNIV passes into your breast milk. Talk to your doctor about the best way to feed your baby while taking INTUNIV.
- ketoconazole
- medicines that can affect enzyme metabolism
- high blood pressure medicine
- sedatives
- benzodiazepines
- barbiturates
- antipsychotics
- Take INTUNIV exactly as your doctor tells you.
- Your doctor may change your dose. Do not change your dose of INTUNIV without talking to your doctor.
- Do not stop taking INTUNIV without talking to your doctor.
- Try not to miss your dose of INTUNIV. If you miss a dose of INTUNIV, take the next dose at your regular time. If you miss 2 or more doses, talk to your doctor, as you may need to restart INTUNIV with a lower dose.
- Do not take a double dose to make up for a missed dose.
- INTUNIV should be taken 1 time a day in the morning or in the evening, either alone or in combination with an ADHD stimulant medicine that your doctor may prescribe. Your doctor will tell you when to take INTUNIV and when to take your ADHD stimulant medication.
- INTUNIV should be swallowed whole with a small amount of water, milk, or other liquid.
- Do not crush, chew, or break INTUNIV. Tell your doctor if you cannot swallow INTUNIV whole.
- Do not take INTUNIV with a high-fat meal.
- Your doctor will check your blood pressure and heart rate while you take INTUNIV.
- If you take too much INTUNIV, call your local Poison Control Center at 1-800-222-1222 or go to the nearest emergency room right away.
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how INTUNIV affects you. INTUNIV can slow your thinking and motor skills.
- Do not drink alcohol or take other medicines that make you sleepy or dizzy while taking INTUNIV until you talk with your doctor. INTUNIV taken with alcohol or medicines that cause sleepiness or dizziness may make your sleepiness or dizziness worse.
- Do not become dehydrated or overheated. This may increase your chance of having low blood pressure or fainting while taking INTUNIV.
- Do not suddenly stop INTUNIV. Tell your healthcare provider if you have been vomiting and cannot take INTUNIV, you may be at risk for rebound hypertension.
- low blood pressure
- low heart rate
- fainting
- sleepiness
- increased blood pressure and heart rate after suddenly stopping INTUNIV (rebound hypertension). Suddenly stopping INTUNIV can cause increased blood pressure and heart rate and other withdrawal symptoms such as headache, confusion, nervousness, agitation, and tremors. If these symptoms continue to get worse and are left untreated, it could lead to a very serious condition including very high blood pressure, feeling very sleepy or tired, severe headache, vomiting, vision problems, seizures.
- sleepiness
- tiredness
- trouble sleeping
- low blood pressure
- nausea
- stomach pain
- dizziness
- dry mouth
- irritability
- vomiting
- slow heart rate
- Store INTUNIV between 68°F to 77°F (20°C to 25°C)
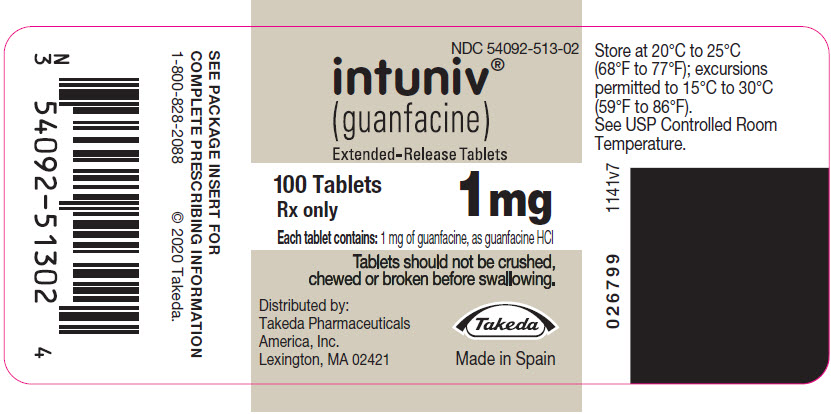
(guanfacine)
Extended-Release Tablets
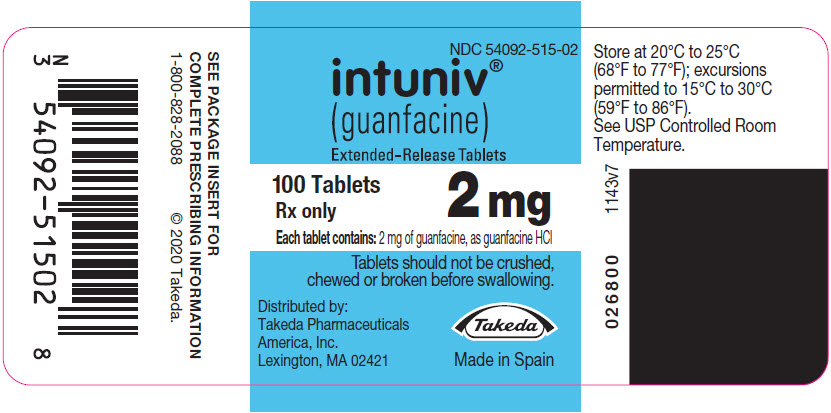
(guanfacine)
Extended-Release Tablets
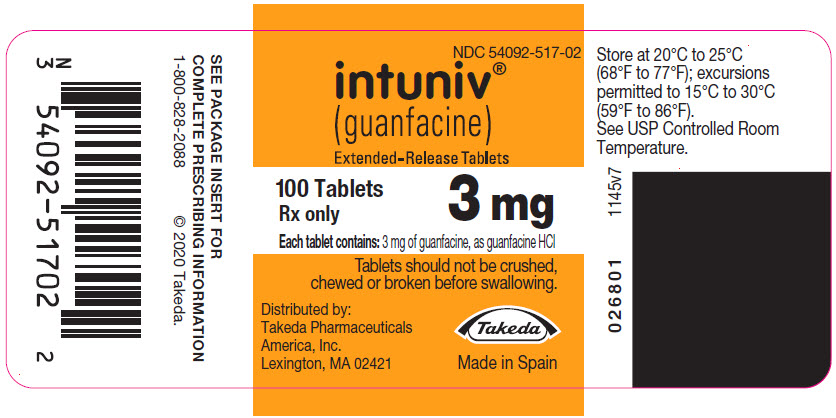
(guanfacine)
Extended-Release Tablets
(guanfacine)
Extended-Release Tablets

