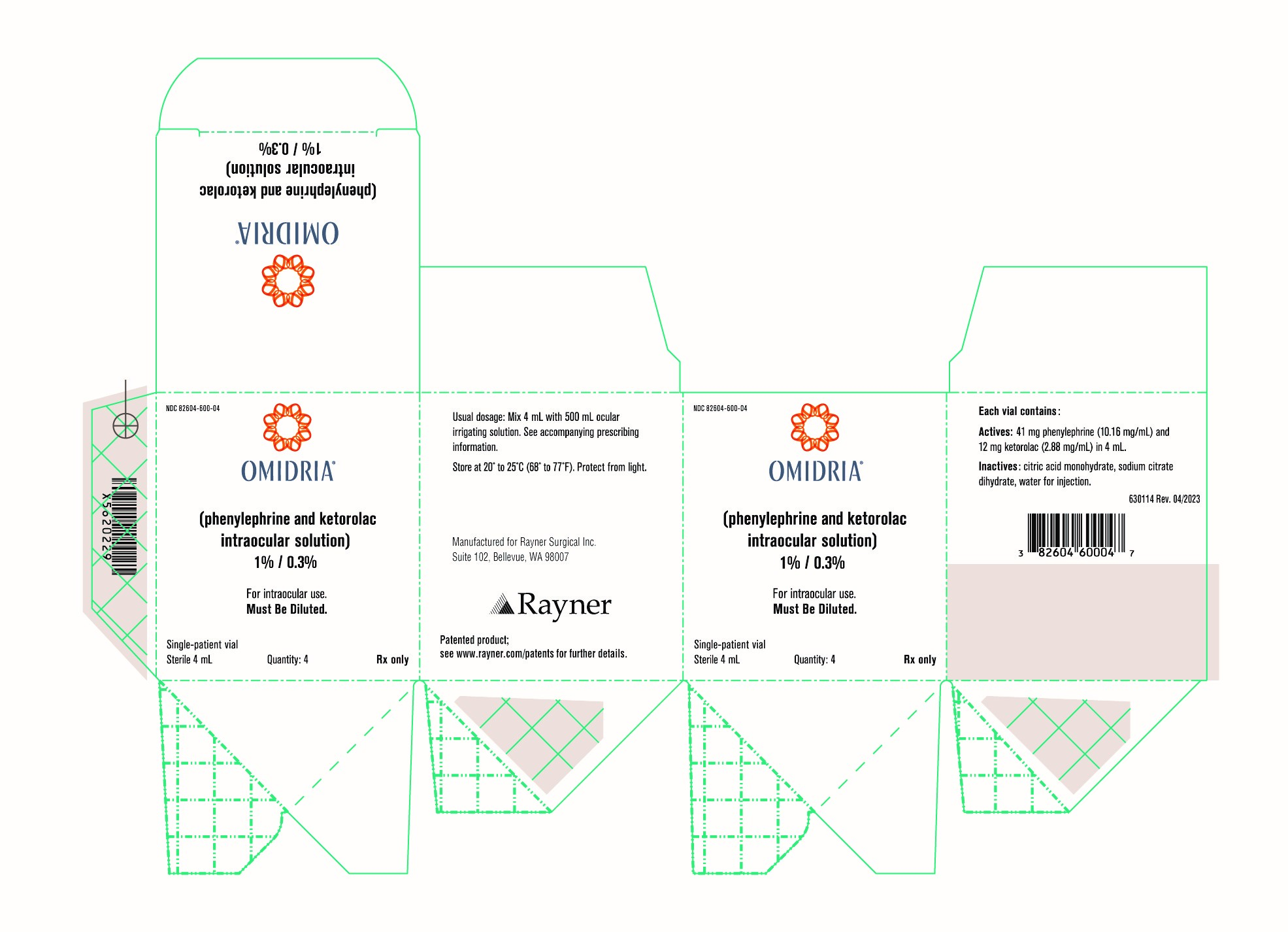
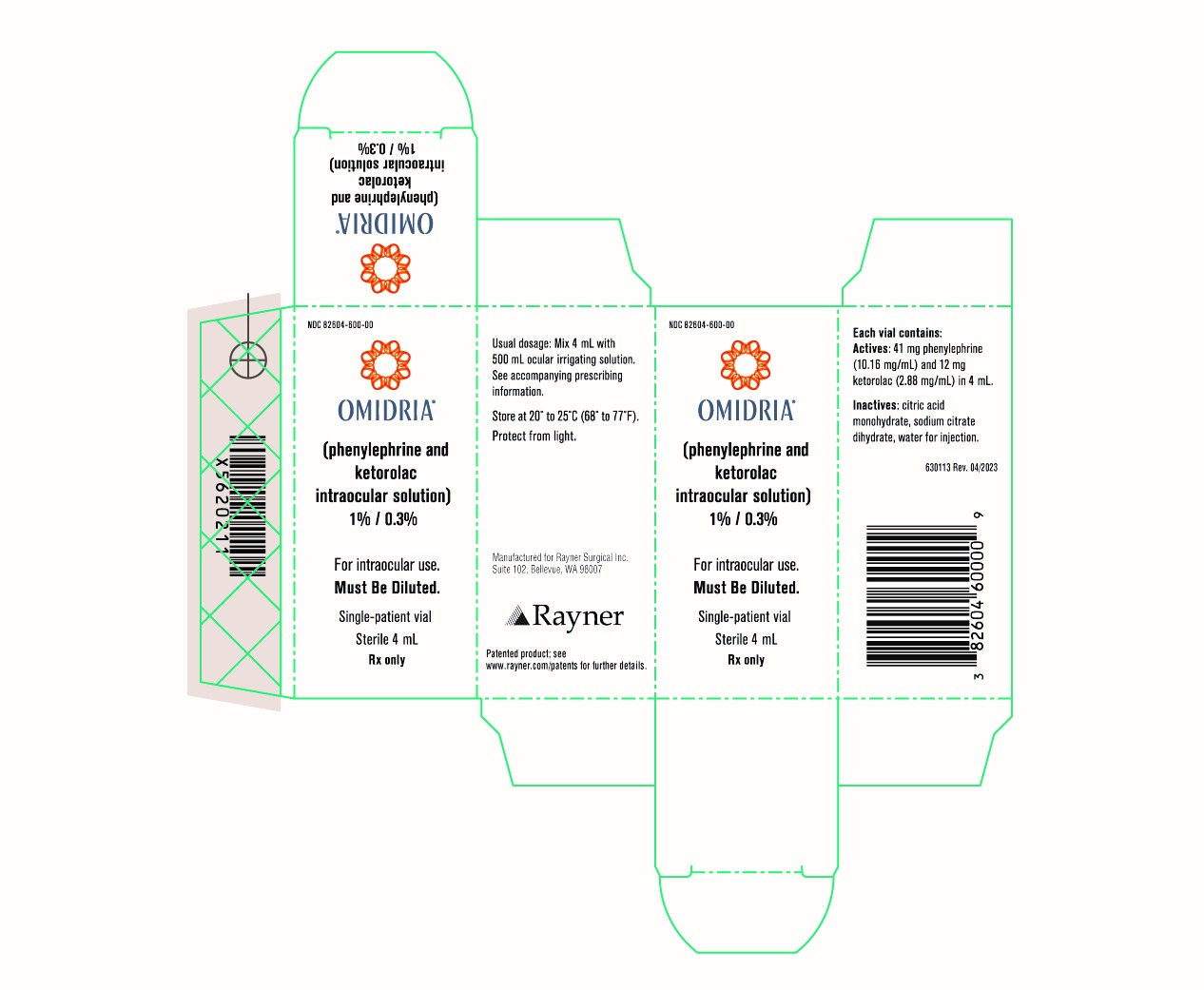
Omidria
What is Omidria (Ketorolac)?
Approved To Treat
Related Clinical Trials
Summary: Gestational Diabetes Mellitus (GDM) currently affects approximately 14% of all pregnancies worldwide. Importantly, the health-related consequences of GDM extend well beyond pregnancy, such that women with a history of GDM have a 40% increased risk of cerebrovascular diseases and a 67% increased risk of dementia, compared to women with a history of uncomplicated pregnancy. Women with a history of G...
Summary: Ketorolac is a medication often used to relieve pain after surgery. In the past, infertility doctors have been cautious about using ketorolac after egg retrieval for patients planning a fresh embryo transfer (usually done 5 days later). The concern was that ketorolac might increase the risk of bleeding or reduce the chances of the embryo implanting in the uterus. This concern comes from how ketoro...
Summary: The objective of this study is to conduct a randomized clinical trial to evaluate an opioid versus an opioid-free pathway of perioperative use of ketamine, ketorolac, and IV acetaminophen followed by the postoperative use of ketorolac, and oral acetaminophen for pain associated with robotic-assisted radical prostatectomy. Escalation to use of opioid treatment for the opioid-free constituents will ...
Related Latest Advances
Brand Information
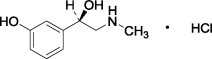
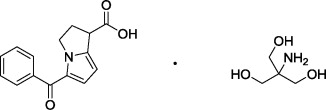
Patented product; see www.rayner.com/patents for further details.
640069