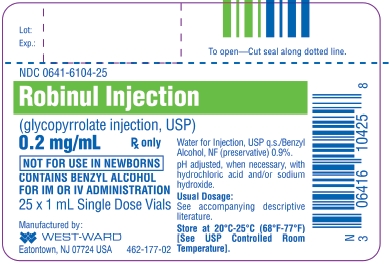
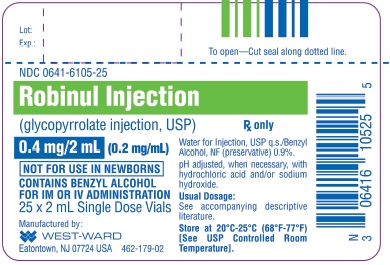
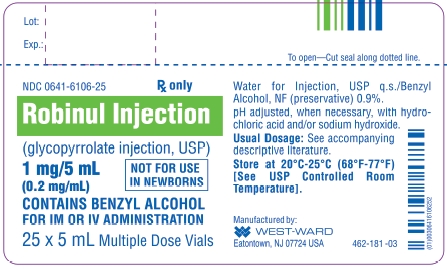
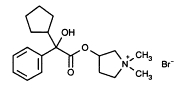
Glycopyrrolate
What is Robinul (Glycopyrrolate)?
Related Clinical Trials
Summary: This study will assess the safety and efficacy of fixed dose combinations of ensifentrine with two different glycopyrrolate dose levels compared to placebo and to the individual components of the fixed dose combinations, each administered twice a day via standard jet nebulizer, in adult subjects with chronic obstructive pulmonary disease (COPD).
Summary: The CHOROS pooled analysis is a retrospective secondary data use analysis of integrated individual participant data from a series of planned and on-going primary prospective, non-interventional, multi-center studies sponsored by AstraZeneca and conducted in the pulmonary/primary care practitioner setting in multiple countries and may include data from the following countries: United Kingdom, Germa...
Summary: This study will assess the pharmacokinetics (PK), pharmacodynamics (PD) and safety of ensifentrine and glycopyrrolate fixed dose (FDC) product, the individual components of the FDC (ensifentrine and glycopyrrolate, each in the FDC formulation), ensifentrine 1.5 mg in the FDC formulation and ensifentrine 3 mg in the marketed formulation each administered via a standard jet nebulizer, in adult parti...
Related Latest Advances
Brand Information
Eatontown, NJ 07724 USA