Lytgobi
What is Lytgobi (Futibatinib)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The purpose of the study is to learn from the real world practice of prescribing targeted therapies to patients with advanced cancer whose tumor harbors a genomic variant known to be a drug target or to predict sensitivity to a drug. NOTE: Due to character limits, the arms section does NOT include all TAPUR Study relevant biomarkers. For additional information, contact TAPUR@asco.org, or if a pati...
Summary: This proof-of-concept platform trial is designed to cover the targeting of several survival pathways in oncogenesis that are currently not adequately employed for pediatric patients in Europe (Geoerger 2017; Geoerger 2019). The aims of the trial are: 1. To determine the recommended phase II dose (RP2D) of a specific anticancer agent and/or a relevant combination in a pediatric population, to docum...
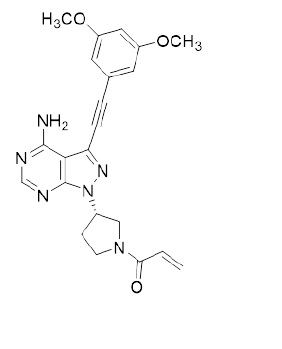
Summary: This is an open-label, multinational, randomized Phase 2 study confirming the clinical benefit of 20 mg futibatinib and evaluating the safety and efficacy of 16 mg futibatinib in previously treated CCA harboring FGFR2 gene fusions and other rearrangements.
Related Latest Advances
Brand Information
- Ocular Toxicity
- Hyperphosphatemia and Soft Tissue Mineralization
- 20 mg daily dose: Each carton contains 1 blister card containing a 7-day supply (35 tablets; 4 mg futibatinib per tablet). [NDC-64842-0120-6]
- 20 mg daily dose: Each carton contains 1 blister card containing a 7-day supply (14 tablets; 7 tablets each of 4 mg and 16 mg futibatinib per tablet). [NDC-64842-0120-8]
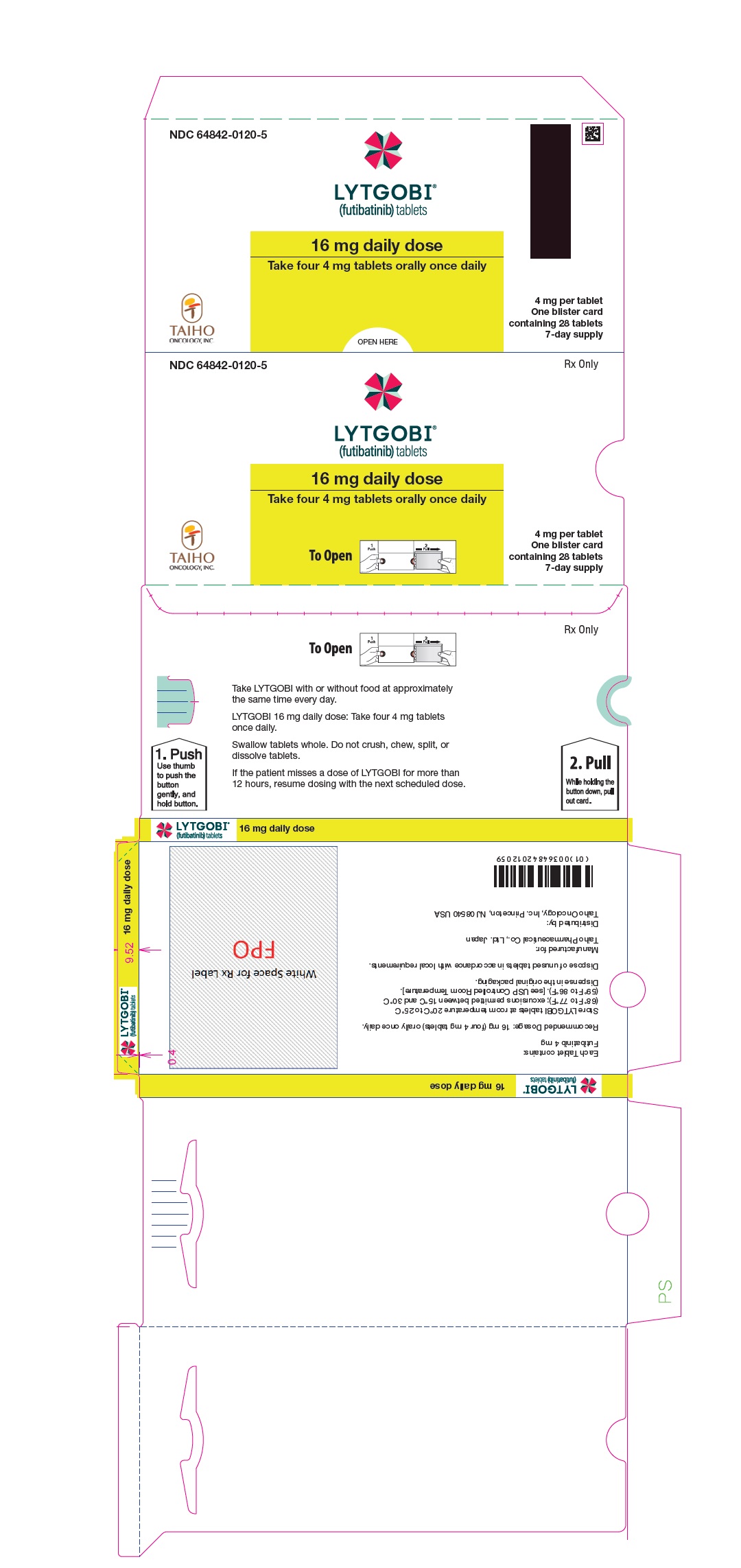
- 16 mg daily dose: Each carton contains 1 blister card containing a 7-day supply (28 tablets; 4 mg futibatinib per tablet). [NDC-64842-0120-5]
- 16 mg daily dose: Each carton contains 1 blister card containing a 7-day supply (7 tablets; 16 mg futibatinib per tablet). [NDC-64842-0120-7]
- 12 mg daily dose: Each carton contains 1 blister card containing a 7-day supply (21 tablets; 4 mg futibatinib per tablet). [NDC-64842-0120-4]
- Advise females to inform their healthcare provider if they are pregnant or become pregnant. Inform female patients of the risk to a fetus and potential loss of pregnancy
- Advise females of reproductive potential to use effective contraception while on LYTGOBI and for 1 week after the last dose
- Advise males with female partners of reproductive potential or who are pregnant to use effective contraception during treatment and for 1 week after receiving the last dose of LYTGOBI
- Advise patients not to breastfeed during treatment with LYTGOBI and for 1 week after the last dose
- Instruct patients to not crush, chew, split or dissolve tablets.
- Instruct patients if they miss a dose by 12 or more hours or if they vomit after taking a dose, resume dosing with the next scheduled dose. Extra tablets should not be taken to make up for the missed dose

(futibatinib) tablets

(futibatinib) tablets
16 mg per tablet (7 tablets)

(futibatinib) tablets

(futibatinib) tablets