Brand Name
Livdelzi
Generic Name
Seladelpar
View Brand Information FDA approval date: August 14, 2024
Form: Capsule
What is Livdelzi (Seladelpar)?
LIVDELZI is indicated for the treatment of primary biliary cholangitis in combination with ursodeoxycholic acid in adults who have had an inadequate response to UDCA, or as monotherapy in patients unable to tolerate UDCA. This indication is approved under accelerated approval based on a reduction of alkaline phosphatase . Improvement in survival or prevention of liver decompensation events have not been demonstrated. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial. LIVDELZI is a peroxisome proliferator-activated receptor -delta agonist indicated for the treatment of primary biliary cholangitis in combination with ursodeoxycholic acid in adults who have an inadequate response to UDCA, or as monotherapy in patients unable to tolerate UDCA. This indication is approved under accelerated approval based on a reduction of alkaline phosphatase . Improvement in survival or prevention of liver decompensation events have not been demonstrated. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial. Limitations of Use Use of LIVDELZI is not recommended in patients who have or develop decompensated cirrhosis .
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
AFFIRM: A Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Effect of Seladelpar on Clinical Outcomes in Patients With Primary Biliary Cholangitis (PBC) and Compensated Cirrhosis
Summary: To Evaluate the Effect of Seladelpar on Clinical Outcomes in Patients with Primary Biliary Cholangitis (PBC) and Compensated Cirrhosis.
Related Latest Advances
Brand Information
Livdelzi (SELADELPAR LYSINE)
1INDICATIONS AND USAGE
LIVDELZI is indicated for the treatment of primary biliary cholangitis (PBC) in combination with ursodeoxycholic acid (UDCA) in adults who have had an inadequate response to UDCA, or as monotherapy in patients unable to tolerate UDCA.
This indication is approved under accelerated approval based on a reduction of alkaline phosphatase (ALP)
2DOSAGE FORMS AND STRENGTHS
Capsules: 10 mg, opaque, hard gelatin capsules, size 1, with light gray opaque body and a dark blue opaque cap, printed with "CBAY" on the cap and "10" on the body.
3CONTRAINDICATIONS
None.
4ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in labeling:
- Fractures
- Liver Test Abnormalities
4.1Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In Trial 1, 193 patients were randomized to receive either LIVDELZI 10 mg (N=128) or placebo (N=65) once daily for 12 months
5OVERDOSAGE
PBC patients who received 5-times the recommended dosage or 20-times the recommended dosage of LIVDELZI experienced an increase in liver transaminases, muscle pain, and/or elevations in creatine phosphokinase, which resolved upon LIVDELZI discontinuation
There is no specific treatment for overdose with LIVDELZI. General supportive care of the patient is indicated, as appropriate. If indicated, elimination of unabsorbed drug should be achieved by emesis or gastric lavage; usual precautions should be observed to maintain the airway. Because seladelpar is highly bound to plasma proteins, hemodialysis should not be considered.
6DESCRIPTION
LIVDELZI capsules contain seladelpar lysine, a peroxisome proliferator-activated receptor (PPAR)-delta (δ) agonist. Seladelpar is a single enantiomer of the R-configuration and is present as a lysine dihydrate salt. Seladelpar lysine dihydrate is a white to off-white powder with a molecular formula of C
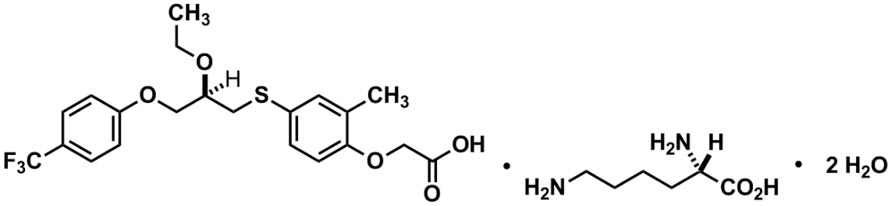
LIVDELZI (seladelpar) capsules are supplied in a 10 mg strength for oral administration. Each capsule contains 14.1 mg of seladelpar lysine and the following inactive ingredients: butylated hydroxytoluene, colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, mannitol, microcrystalline cellulose, and hard gelatin shells.
The light gray opaque (body)/dark blue opaque (cap) capsule shells contain gelatin, titanium dioxide, black iron oxide, yellow iron oxide, red iron oxide and the colorant FD&C Blue #2.
7CLINICAL STUDIES
The efficacy of LIVDELZI was evaluated in Trial 1 (NCT04620733), a 12-month, randomized, double-blind, placebo-controlled trial. The study included 193 adult patients with PBC with an inadequate response or intolerance to UDCA. Patients were included in the trial if their ALP was greater than or equal to 1.67-times the ULN and total bilirubin (TB) was less than or equal to 2-times the ULN. Patients were excluded from the trial if they had other chronic liver diseases, clinically important hepatic decompensation including portal hypertension with complications, or cirrhosis with complications (e.g., Model for End Stage Liver Disease [MELD] score of 12 or greater, known esophageal varices or history of variceal bleeds, history of hepatorenal syndrome).
Patients were randomized to receive LIVDELZI 10 mg (N=128) or placebo (N=65) once daily for 12 months. LIVDELZI or placebo was administered in combination with UDCA in 181 (94%) patients during the trial, or as a monotherapy in 12 (6%) patients who were unable to tolerate UDCA.
8PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
9PRINCIPAL DISPLAY PANEL - 10 mg Capsule Bottle Label
NDC 61958-3301-1
Livdelzi
seladelpar
seladelpar
10 mg
For Oral Use Only
Rx only
GILEAD
GILEAD
10PRINCIPAL DISPLAY PANEL - 10 mg Capsule Bottle Label
NDC 61958-3301-2
Livdelzi
seladelpar
seladelpar
10 mg
For Oral Use Only
Rx only
GILEAD
GILEAD

