Motegrity
What is Motegrity (Prucalopride)?
For many people, chronic constipation isn’t just an inconvenience, it can be a daily struggle that affects comfort, mood, and quality of life. When lifestyle changes and over-the-counter remedies don’t provide enough relief, prescription options like Motegrity (prucalopride) can make a meaningful difference.
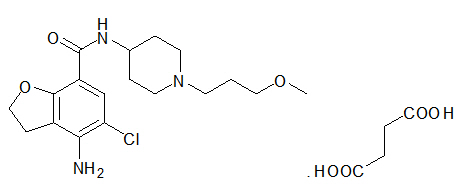
Motegrity is a prescription medication used to treat chronic idiopathic constipation (CIC) in adults. “Idiopathic” means the constipation has no identifiable underlying cause. Motegrity belongs to a class of drugs known as selective serotonin type 4 (5-HT4) receptor agonists, which work by stimulating natural bowel movement activity.
Approved by the U.S. Food and Drug Administration (FDA) in 2018, Motegrity represents a newer, targeted approach to treating chronic constipation compared to older stimulant laxatives. It’s designed for people who have not found consistent relief from diet, hydration, or other medications.
What does Motegrity do?
Motegrity helps increase the frequency and predictability of bowel movements in adults with chronic idiopathic constipation. People with CIC often experience fewer than three bowel movements per week, accompanied by straining, bloating, and discomfort.
By improving bowel motility, the natural contractions of the colon, Motegrity allows stool to move through the digestive tract more efficiently. Patients taking Motegrity typically experience:
- More regular bowel movements
- Less abdominal discomfort and bloating
- A greater sense of complete evacuation after using the bathroom
In clinical studies, significantly more patients achieved three or more spontaneous bowel movements per week compared with those taking a placebo (FDA, 2018). These improvements often lead to better overall comfort and day-to-day functioning.
Motegrity is not a quick-acting laxative. Instead, it supports the body’s natural rhythm, making it a suitable long-term therapy for those with persistent constipation.
How does Motegrity work?
Motegrity contains prucalopride, which works by activating serotonin (5-HT4) receptors in the digestive tract. These receptors help regulate the muscle contractions that move stool through the colon.
In simple terms, prucalopride stimulates the bowel muscles to contract in a coordinated, wave-like pattern known as peristalsis. This action promotes regular bowel movements without forcing the colon to work unnaturally hard, as some stimulant laxatives do.
Clinically, this mechanism matters because many people with chronic idiopathic constipation have sluggish or uncoordinated colon movement. By enhancing natural motility rather than merely softening stool, Motegrity addresses one of the root causes of chronic constipation.
Unlike older 5-HT4 agonists, prucalopride is highly selective, meaning it primarily acts in the gut and has a lower risk of cardiovascular side effects, which were a concern with earlier drugs in this class (NIH, 2023).
Motegrity side effects
While Motegrity is generally well tolerated, it can cause side effects in some patients. Most are mild and temporary, especially during the first few days of treatment as the body adjusts.
Common side effects include:
- Headache
- Nausea
- Abdominal pain or cramps
- Diarrhea
- Dizziness or fatigue
Less common but potentially serious side effects may include:
- Mood changes or new or worsening depression
- Suicidal thoughts (rare but reported)
- Allergic reactions such as swelling, rash, or difficulty breathing
Patients and caregivers should monitor for any mood or behavioral changes, especially early in treatment. Those with a history of depression or suicidal thoughts should inform their doctor before starting Motegrity.
Seek immediate medical attention if you experience symptoms of a severe allergic reaction or unusual mood changes.
Avoid Motegrity if you have: intestinal perforation or obstruction, severe inflammatory digestive conditions (e.g., Crohn’s, ulcerative colitis), or allergies to prucalopride or its inactive ingredients.
Overall, the majority of patients tolerate Motegrity well, and most side effects lessen with continued use or dose adjustments made under medical supervision.
Motegrity dosage
Motegrity is a daily oral tablet, taken with or without food, for long-term use. Dosage is based on age, kidney function, and health, with lower doses potentially needed for severe kidney impairment. Consistent daily use is crucial for effectiveness.
Follow-up visits assess bowel movement frequency/consistency, monitor side effects/emotional well-being, and adjust dosage.
Older adults and those with kidney issues should use Motegrity cautiously due to kidney clearance. If a dose is missed, take it when remembered, unless it’s near the next dose to avoid doubling.
Does Motegrity have a generic version?
As of 2025, no generic version of Motegrity (prucalopride) is available in the United States. It remains a brand-name medication manufactured by Takeda Pharmaceuticals. However, international versions may exist in other markets.
Generic prucalopride exists in Europe and Canada. Once an FDA-approved generic is available in the U.S., it will be safe, effective, bioequivalent, and cheaper. For affordability concerns, patients can explore savings programs with their healthcare provider or pharmacist.
Conclusion
Motegrity (prucalopride) offers a modern, targeted solution for adults struggling with chronic idiopathic constipation, a condition that can significantly impact daily comfort and quality of life. By gently stimulating the natural muscle activity of the colon, it helps restore regular, predictable bowel movements without relying on harsh stimulant laxatives.
While side effects like headache or nausea can occur, most are manageable, and serious risks are rare with proper use. Each patient’s experience is unique. Discuss symptoms, mental health history, and other medications with your doctor before starting Motegrity. Under medical supervision, Motegrity can provide lasting relief, improve digestive health, and enhance well-being.
References
- U.S. Food and Drug Administration (FDA). (2018). FDA approves Motegrity (prucalopride) for chronic idiopathic constipation in adults. Retrieved from https://www.fda.gov
- Mayo Clinic. (2024). Prucalopride (oral route) description and precautions. Retrieved from https://www.mayoclinic.org
- MedlinePlus. (2024). Prucalopride: Drug information. National Library of Medicine. Retrieved from https://medlineplus.gov
- National Institutes of Health (NIH). (2023). 5-HT4 receptor agonists and gastrointestinal motility. Retrieved from https://www.nih.gov
Approved To Treat
Top Global Experts
There are no experts for this drug
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
- 1 mg prucalopride: White to off-white, round, biconvex film-coated tablet debossed with "PRU 1" on one side and no debossing on the other side.
- 2 mg prucalopride: Pink, round, biconvex film-coated tablet debossed with "PRU 2" on one side and no debossing on the other side.
- A history of hypersensitivity to MOTEGRITY. Reactions including dyspnea, rash, pruritus, urticaria, and facial edema have been observed
- Intestinal perforation or obstruction due to structural or functional disorder of the gut wall, obstructive ileus, severe inflammatory conditions of the intestinal tract such as Crohn's disease, ulcerative colitis, and toxic megacolon/megarectum.
- Lumpy or hard stools
- Sensation of incomplete evacuation
- Straining at defecation
- NDC 54092-546-01: HDPE bottle of 30 tablets, with child-resistant closure.
- NDC 54092-547-01: HDPE bottle of 30 tablets, with child-resistant closure.
- Suicidal Ideation and Behavior: Inform patients, their caregivers, and family members that suicidal ideation and behavior, self-injurious ideation as well as new onset or worsening depression have been reported in patients treated with MOTEGRITY. Advise them to be aware of any unusual changes in mood or behavior, new onset or worsening of depression, or the emergence of suicidal thoughts or behavior. Instruct patients, caregivers, and family members to discontinue MOTEGRITY immediately and contact their healthcare provider if any of these symptoms occur [see .
- Pregnancy: Advise patients that there is a pregnancy registry that monitors pregnancy outcomes in women exposed to MOTEGRITY during pregnancy

