Generic Name
Lubiprostone
Brand Names
Amitiza, Amitza
FDA approval date: January 31, 2006
Classification: Chloride Channel Activator
Form: Capsule
What is Amitiza (Lubiprostone)?
Lubiprostone capsules is a chloride channel activator indicated for the treatment of: chronic idiopathic constipation in adults.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Amitiza (lubiprostone)
1DOSAGE FORMS AND STRENGTHS
Amitiza is available as an oval, gelatin capsule containing 8 mcg or 24 mcg of lubiprostone.
- 8 mcg capsules are pink and are printed with "SPI" on one side
- 24 mcg capsules are orange and are printed with "SPI" on one side
2CONTRAINDICATIONS
Amitiza is contraindicated in patients with known or suspected mechanical gastrointestinal obstruction
3ADVERSE REACTIONS
The following adverse reactions are described below and elsewhere in labeling:
- Nausea
- Diarrhea
- Syncope and Hypotension
- Dyspnea
3.1Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
During clinical development of Amitiza for CIC, OIC, and IBS-C, 1648 patients were treated with Amitiza for 6 months and 710 patients were treated for 1 year (not mutually exclusive).
3.2Postmarketing Experience
The following additional adverse reactions have been identified during post-approval use of Amitiza. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiovascular: syncope and/or hypotension [see , tachycardia
Gastrointestinal: ischemic colitis
General: asthenia
Immune System: hypersensitivity reactions including rash, swelling, and throat tightness malaise
Muscoskeletal: muscle cramps or muscle spasms.
4OVERDOSAGE
There have been six reports of overdosage with Amitiza during clinical development. Of these six cases, only two subjects reported adverse events: one reported vomiting, diarrhea and stomach ache after taking 168 to 192 mcg of Amitiza, and another reported diarrhea and a joint injury on the day of overdose after taking 36 mcg of Amitiza. Adverse reactions that occurred in at least 1% of healthy subjects given a single oral dose of 144 mcg of Amitiza (6 times the highest recommended dose) in a cardiac repolarization study included nausea (45%), diarrhea (35%), vomiting (27%), dizziness (14%), headache (12%), abdominal pain (8%), flushing/hot flash (8%), retching (8%), dyspnea (4%), pallor (4%), stomach discomfort (4%), anorexia (2%), asthenia (2%), chest discomfort (2%), dry mouth (2%), hyperhidrosis (2%), and syncope (2%).
5DESCRIPTION
Amitiza (lubiprostone) is a chloride channel activator for oral use.
The chemical name for lubiprostone is (–)-7-[(2
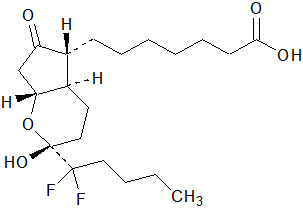
Lubiprostone drug substance occurs as white, odorless crystals or crystalline powder, is very soluble in ether and ethanol, and is practically insoluble in hexane and water. Amitiza is available as an imprinted, oval, soft gelatin capsule in two strengths. Pink capsules contain 8 mcg of lubiprostone and the following inactive ingredients: ferric oxide, gelatin, medium-chain triglycerides, purified water, sorbitol, and titanium dioxide. Orange capsules contain 24 mcg of lubiprostone and the following inactive ingredients: D&C Yellow #10, FD&C Red #40, gelatin, medium-chain triglycerides, purified water, and sorbitol.
6HOW SUPPLIED/STORAGE AND HANDLING
Amitiza is available as an oval, soft gelatin capsule containing 8 mcg or 24 mcg of lubiprostone with "SPI" printed on one side. Amitiza is available as follows:
8 mcg pink capsule
- Bottles of 60 (NDC 23635-508-60)
24 mcg orange capsule
- Bottles of 60 (NDC 23635-524-60)
7PATIENT COUNSELING INFORMATION
Administration Instructions
- Instruct patients to take Amitiza orally with food and water to reduce the occurrence of nausea
- Swallow capsules whole and do not break apart or chew.
- Physicians and patients should periodically assess the need for continued therapy.
8PRINCIPAL DISPLAY PANEL - 8 mcg Capsule Bottle Label
Rx Only
60 Capsules
AMITIZA
NDC 23635-508-60
SUCAMPO
See package insert for complete prescribing information.
Mallinckrodt™
9PRINCIPAL DISPLAY PANEL - 24 mcg Capsule Bottle Label
Rx Only
60 Capsules
AMITIZA
NDC 23635-524-60
SUCAMPO
See package insert for complete prescribing information.
Mallinckrodt™
