Generic Name
Dasatinib
Brand Names
Sprycel, Phyrago
FDA approval date: June 27, 2006
Classification: Kinase Inhibitor
Form: Tablet
What is Sprycel (Dasatinib)?
Dasatinib tablets are indicated for the treatment of adult patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase., chronic, accelerated, or myeloid or lymphoid blast phase Ph+ CML with resistance or intolerance to prior therapy including imatinib., Philadelphia chromosome-positive acute lymphoblastic leukemia with resistance or intolerance to prior therapy. Dasatinib tablets are indicated for the treatment of pediatric patients 1 year of age and older with, Ph+ CML in chronic phase., newly diagnosed Ph+ ALL in combination with chemotherapy. Dasatinib is a kinase inhibitor indicated for the treatment of, newly diagnosed adults with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase. , adults with chronic, accelerated, or myeloid or lymphoid blast phase Ph+ CML with resistance or intolerance to prior therapy including imatinib. , adults with Philadelphia chromosome-positive acute lymphoblastic leukemia with resistance or intolerance to prior therapy. , pediatric patients 1 year of age and older with Ph+ CML in chronic phase. , pediatric patients 1 year of age and older with newly diagnosed Ph+ ALL in combination with chemotherapy.
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
SPRYCEL (dasatinib)
1INDICATIONS AND USAGE
SPRYCEL (dasatinib) is indicated for the treatment of adult patients with
- newly diagnosed Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia (CML) in chronic phase.
- chronic, accelerated, or myeloid or lymphoid blast phase Ph+ CML with resistance or intolerance to prior therapy including imatinib.
- Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) with resistance or intolerance to prior therapy.
SPRYCEL (dasatinib) is indicated for the treatment of pediatric patients 1 year of age and older with
- Ph+ CML in chronic phase.
- newly diagnosed Ph+ ALL in combination with chemotherapy.
2DOSAGE FORMS AND STRENGTHS
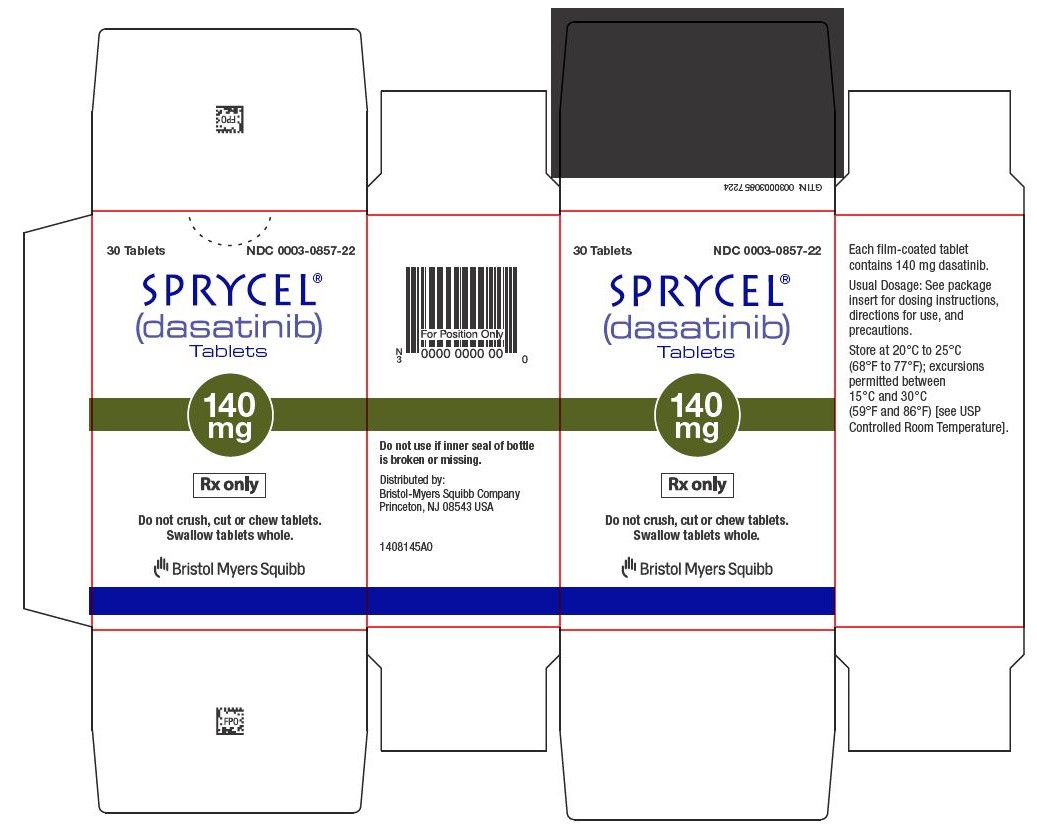
SPRYCEL (dasatinib) Tablets are available as 20-mg, 50-mg, 70-mg, 80-mg, 100-mg, and 140-mg white to off-white, biconvex, film-coated tablets.
3CONTRAINDICATIONS
None.
4ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling:
- Myelosuppression
- Bleeding-related events
- Fluid retention
- Cardiovascular toxicity
- Pulmonary arterial hypertension
- QT prolongation
- Severe dermatologic reactions
- Tumor lysis syndrome
- Effects on growth and development in pediatric patients
- Hepatotoxicity
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to SPRYCEL administered as single-agent therapy at all doses tested in clinical studies (n=2809), including 324 adult patients with newly diagnosed chronic phase CML, 2388 adult patients with imatinib-resistant or -intolerant chronic or advanced phase CML or Ph+ ALL, and 97 pediatric patients with chronic phase CML. The median duration of therapy in a total of 2712 adult patients was 19.2 months (range 0 to 93.2 months). In a randomized trial in patients with newly diagnosed chronic phase CML, the median duration of therapy was approximately 60 months. The median duration of therapy in 1618 adult patients with chronic phase CML was 29 months (range 0 to 92.9 months).
The median duration of therapy in 1094 adult patients with advanced phase CML or Ph+ ALL was 6.2 months (range 0 to 93.2 months).
In two non-randomized trials in 97 pediatric patients with chronic phase CML (51 patients newly diagnosed and 46 patients resistant or intolerant to previous treatment with imatinib), the median duration of therapy was 51.1 months (range 1.9 to 99.6 months).
In the overall population of 2712 adult patients, 88% of patients experienced adverse reactions at some time and 19% experienced adverse reactions leading to treatment discontinuation.
In the randomized trial in adult patients with newly diagnosed chronic phase CML, drug was discontinued for adverse reactions in 16% of patients with a minimum of 60 months of follow-up. After a minimum of 60 months of follow-up, the cumulative discontinuation rate was 39%. Among the 1618 patients with chronic phase CML, drug-related adverse reactions leading to discontinuation were reported in 329 (20.3%) patients; among the 1094 patients with advanced phase CML or Ph+ ALL, drug-related adverse reactions leading to discontinuation were reported in 191 (17.5%) patients.
Among the 97 pediatric subjects, drug-related adverse reactions leading to discontinuation were reported in 1 patient (1%).
Adverse reactions reported in ≥10% of adult patients, and other adverse reactions of interest, in a randomized trial in patients with newly diagnosed chronic phase CML at a median follow-up of approximately 60 months are presented in Table 6.
Adverse reactions reported in ≥10% of adult patients treated at the recommended dose of 100 mg once daily (n=165), and other adverse reactions of interest, in a randomized dose-optimization trial of patients with chronic phase CML resistant or intolerant to prior imatinib therapy at a median follow-up of approximately 84 months are presented in Table 8.
Adverse reactions reported in ≥10% of pediatric patients at a median follow-up of approximately 51.1 months are presented in Table 11.
Drug-related serious adverse reactions (SARs) were reported for 16.7% of adult patients in the randomized trial of patients with newly diagnosed chronic phase CML. Serious adverse reactions reported in ≥5% of patients included pleural effusion (5%).
Drug-related SARs were reported for 26.1% of patients treated at the recommended dose of 100 mg once daily in the randomized dose-optimization trial of adult patients with chronic phase CML resistant or intolerant to prior imatinib therapy. Serious adverse reactions reported in ≥5% of patients included pleural effusion (10%).
Drug-related SARs were reported for 14.4% of pediatric patients.
4.1.1Chronic Myeloid Leukemia (CML)
Adverse reactions (excluding laboratory abnormalities) that were reported in at least 10% of adult patients are shown in Table 6 for newly diagnosed patients with chronic phase CML and Tables 8 and 10 for CML patients with resistance or intolerance to prior imatinib therapy.
A comparison of cumulative rates of adverse reactions reported in ≥10% of patients with minimum follow-up of 1 and 5 years in a randomized trial of newly diagnosed patients with chronic phase CML treated with SPRYCEL are shown in Table 7.
At 60 months, there were 26 deaths in dasatinib-treated patients (10.1%) and 26 deaths in imatinib-treated patients (10.1%); 1 death in each group was assessed by the investigator as related to study therapy.
Cumulative rates of selected adverse reactions that were reported over time in patients treated with the 100 mg once daily recommended starting dose in a randomized dose-optimization trial of imatinib-resistant or -intolerant patients with chronic phase CML are shown in Table 9.
Adverse reactions associated with bone growth and development were reported in 5 (5.2%) of pediatric patients with chronic phase CML
4.1.1.1Laboratory Abnormalities
Myelosuppression was commonly reported in all patient populations. The frequency of Grade 3 or 4 neutropenia, thrombocytopenia, and anemia was higher in patients with advanced phase CML than in chronic phase CML (Tables 12 and 13). Myelosuppression was reported in patients with normal baseline laboratory values as well as in patients with pre-existing laboratory abnormalities.
In patients who experienced severe myelosuppression, recovery generally occurred following dose interruption or reduction; permanent discontinuation of treatment occurred in 2% of adult patients with newly diagnosed chronic phase CML and 5% of adult patients with resistance or intolerance to prior imatinib therapy
Grade 3 or 4 elevations of transaminases or bilirubin and Grade 3 or 4 hypocalcemia, hypokalemia, and hypophosphatemia were reported in patients with all phases of CML but were reported with an increased frequency in patients with myeloid or lymphoid blast phase CML. Elevations in transaminases or bilirubin were usually managed with dose reduction or interruption. Patients developing Grade 3 or 4 hypocalcemia during SPRYCEL therapy often had recovery with oral calcium supplementation.
Laboratory abnormalities reported in adult patients with newly diagnosed chronic phase CML are shown in Table 12. There were no discontinuations of SPRYCEL therapy in this patient population due to biochemical laboratory parameters.
Laboratory abnormalities reported in patients with CML resistant or intolerant to imatinib who received the recommended starting doses of SPRYCEL are shown by disease phase in Table 13.
Among adult patients with chronic phase CML with resistance or intolerance to prior imatinib therapy, cumulative Grade 3 or 4 cytopenias were similar at 2 and 5 years including: neutropenia (36% vs 36%), thrombocytopenia (23% vs 24%), and anemia (13% vs 13%).
In the pediatric studies in CML, the rates of laboratory abnormalities were consistent with the known profile for laboratory parameters in adults.
4.1.2Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia (Ph+ ALL) in Adults
A total of 135 adult patients with Ph+ ALL were treated with SPRYCEL in clinical studies. The median duration of treatment was 3 months (range 0.03–31 months). The safety profile of patients with Ph+ ALL was similar to those with lymphoid blast phase CML. The most frequently reported adverse reactions included fluid retention events, such as pleural effusion (24%) and superficial edema (19%), and gastrointestinal disorders, such as diarrhea (31%), nausea (24%), and vomiting (16%). Hemorrhage (19%), pyrexia (17%), rash (16%), and dyspnea (16%) were also frequently reported. Serious adverse reactions reported in ≥5% of patients included pleural effusion (11%), gastrointestinal bleeding (7%), febrile neutropenia (6%), and infection (5%).
4.1.3Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia (Ph+ ALL) in Pediatric Patients
The safety of SPRYCEL administered continuously in combination with multiagent chemotherapy was determined in a multicohort study of 81 pediatric patients with newly diagnosed Ph+ ALL.
Fatal adverse reactions occurred in 3 patients (4%), all of which were due to infections. Eight (10%) patients experienced adverse reactions leading to treatment discontinuation, including fungal sepsis, hepatotoxicity in the setting of graft versus host disease, thrombocytopenia, CMV infection, pneumonia, nausea, enteritis and drug hypersensitivity.
The most common serious adverse reactions (incidence ≥10%) were pyrexia, febrile neutropenia, mucositis, diarrhea, sepsis, hypotension, infections (bacterial, viral and fungal), hypersensitivity, vomiting, renal insufficiency, abdominal pain, and musculoskeletal pain.
The incidence of common adverse reactions (incidence ≥20%) on study are shown in Table 14:
The incidence of common adverse reactions attributed by the investigator to SPRYCEL (reported at a frequency of ≥10%, all grades and grade 3/4, respectively) on study (N=81), included febrile neutropenia (23%, 23%), nausea (21%, 4%), vomiting (19%, 4%), mucositis (17%, 6%), musculoskeletal pain (17%, 2%), abdominal pain (16%, 5%), diarrhea (16%, 7%), rash (15%, 0%), fatigue (12%, 0%), pyrexia (12%, 6%), and headache (12%, 5%).
CTCAE grade 3/4 laboratory abnormalities in pediatric patients with Ph+ ALL treated with SPRYCEL in combination with chemotherapy are shown in Table 15.
Additional Pooled Data from Clinical Trials
The following additional adverse reactions were reported in adult and pediatric patients (n=2809) in SPRYCEL CML clinical studies and adult patients in Ph+ ALL clinical studies at a frequency of ≥10%, 1%– <10%, 0.1%– <1%, or < 0.1%. These adverse reactions are included based on clinical relevance.
Gastrointestinal Disorders: 1%–<10% – mucosal inflammation (including mucositis/stomatitis), dyspepsia, abdominal distension, constipation, gastritis, colitis (including neutropenic colitis), oral soft tissue disorder; 0.1%–<1% – ascites, dysphagia, anal fissure, upper gastrointestinal ulcer, esophagitis, pancreatitis, gastroesophageal reflux disease; <0.1% – protein losing gastroenteropathy, ileus, acute pancreatitis, anal fistula.
General Disorders and Administration-Site Conditions:≥10% – peripheral edema, face edema; 1%–<10% – asthenia, chest pain, chills; 0.1%–<1% – malaise, other superficial edema, peripheral swelling; <0.1% – gait disturbance.
Skin and Subcutaneous Tissue Disorders: 1%–<10% – alopecia, acne, dry skin, hyperhidrosis, urticaria, dermatitis (including eczema); 0.1%–<1% – pigmentation disorder, skin ulcer, bullous conditions, photosensitivity, nail disorder, neutrophilic dermatosis, panniculitis, palmar-plantar erythrodysesthesia syndrome, hair disorder; <0.1% – leukocytoclastic vasculitis, skin fibrosis.
Respiratory, Thoracic, and Mediastinal Disorders: 1%–<10% – lung infiltration, pneumonitis, cough; 0.1%– <1% – asthma, bronchospasm, dysphonia, pulmonary arterial hypertension; <0.1% – acute respiratory distress syndrome, pulmonary embolism.
Nervous System Disorders: 1%–<10% – neuropathy (including peripheral neuropathy), dizziness, dysgeusia, somnolence; 0.1%–<1% – amnesia, tremor, syncope, balance disorder; <0.1% – convulsion, cerebrovascular accident, transient ischemic attack, optic neuritis, VIIth nerve paralysis, dementia, ataxia.
Blood and Lymphatic System Disorders: 0.1%–<1% – lymphadenopathy, lymphopenia; <0.1% – aplasia pure red cell.
Musculoskeletal and Connective Tissue Disorders: 1%–<10% – muscular weakness, musculoskeletal stiffness; 0.1%–<1% – rhabdomyolysis, tendonitis, muscle inflammation, osteonecrosis, arthritis; <0.1% – epiphyses delayed fusion (reported at 1%–<10% in the pediatric studies), growth retardation (reported at 1%–<10% in the pediatric studies).
Investigations: 1%–<10% – weight increased, weight decreased; 0.1%–<1% – blood creatine phosphokinase increased, gamma-glutamyltransferase increased.
Infections and Infestations: 1%–<10% – pneumonia (including bacterial, viral, and fungal), upper respiratory tract infection/inflammation, herpes virus infection, enterocolitis infection, sepsis (including fatal outcomes [0.2%]).
Metabolism and Nutrition Disorders: 1%–<10% – appetite disturbances, hyperuricemia; 0.1%–<1% – hypoalbuminemia, tumor lysis syndrome, dehydration, hypercholesterolemia; <0.1% – diabetes mellitus.
Cardiac Disorders: 1%–<10% – arrhythmia (including tachycardia), palpitations; 0.1%–<1% – angina pectoris, cardiomegaly, pericarditis, ventricular arrhythmia (including ventricular tachycardia), electrocardiogram T-wave abnormal, troponin increased; <0.1% – cor pulmonale, myocarditis, acute coronary syndrome, cardiac arrest, electrocardiogram PR prolongation, coronary artery disease, pleuropericarditis.
Eye Disorders: 1%–<10% – visual disorder (including visual disturbance, vision blurred, and visual acuity reduced), dry eye; 0.1%–<1% – conjunctivitis, visual impairment, lacrimation increased, <0.1% – photophobia.
Vascular Disorders: 1%–<10% – flushing, hypertension; 0.1%–<1% – hypotension, thrombophlebitis, thrombosis; <0.1% – livedo reticularis, deep vein thrombosis, embolism.
Psychiatric Disorders: 1%–<10% – insomnia, depression; 0.1%–<1% – anxiety, affect lability, confusional state, libido decreased.
Pregnancy, Puerperium, and Perinatal Conditions: <0.1% – abortion.
Reproductive System and Breast Disorders: 0.1%–<1% – gynecomastia, menstrual disorder.
Injury, Poisoning, and Procedural Complications: 1%–<10% – contusion.
Ear and Labyrinth Disorders: 1%–<10% – tinnitus; 0.1%–<1% – vertigo, hearing loss.
Hepatobiliary Disorders: 0.1%–<1% – cholestasis, cholecystitis, hepatitis.
Renal and Urinary Disorders: 0.1%–<1% – urinary frequency, renal failure, proteinuria; <0.1% – renal impairment.
Immune System Disorders: 0.1%–<1% – hypersensitivity (including erythema nodosum).
Endocrine Disorders: 0.1%–<1% – hypothyroidism; <0.1% – hyperthyroidism, thyroiditis.
4.2Postmarketing Experience
The following additional adverse reactions have been identified during post approval use of SPRYCEL. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Infections: hepatitis B virus reactivation
Cardiac disorders: atrial fibrillation/atrial flutter
Respiratory, thoracic, and mediastinal disorders: interstitial lung disease, chylothorax
Skin and subcutaneous tissue disorders: Stevens-Johnson syndrome
Renal and urinary disorders: nephrotic syndrome
Blood and lymphatic system disorders: thrombotic microangiopathy
Hepatobiliary disorders: hepatotoxicity
5OVERDOSAGE
Experience with overdose of SPRYCEL in clinical studies is limited to isolated cases. The highest overdosage of 280 mg per day for 1 week was reported in two patients and both developed severe myelosuppression and bleeding. Since SPRYCEL is associated with severe myelosuppression
Acute overdose in animals was associated with cardiotoxicity. Evidence of cardiotoxicity included ventricular necrosis and valvular/ventricular/atrial hemorrhage at single doses ≥100 mg/kg (600 mg/m
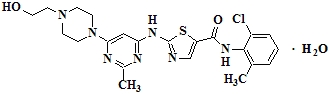
6DESCRIPTION
SPRYCEL (dasatinib) is a kinase inhibitor. The chemical name for dasatinib is N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1-piperazinyl]-2-methyl-4-pyrimidinyl]amino]-5-thiazolecarboxamide, monohydrate. The molecular formula is C
Dasatinib is a white to off-white powder. The drug substance is insoluble in water and slightly soluble in ethanol and methanol.
SPRYCEL tablets are white to off-white, biconvex, film-coated tablets containing dasatinib, with the following inactive ingredients: lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, hydroxypropyl cellulose, and magnesium stearate. The tablet coating consists of hypromellose, titanium dioxide, and polyethylene glycol.
7REFERENCES
1.
8HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
SPRYCEL
Storage
SPRYCEL tablets should be stored at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature].
Handling and Disposal
SPRYCEL is an antineoplastic product. Follow special handling and disposal procedures.
Personnel who are pregnant should avoid exposure to crushed or broken tablets.
SPRYCEL tablets consist of a core tablet, surrounded by a film coating to prevent exposure of healthcare professionals to the active substance. The use of latex or nitrile gloves for appropriate disposal when handling tablets that are inadvertently crushed or broken is recommended, to minimize the risk of dermal exposure.
9PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
10PATIENT INFORMATION SPRYCEL®(Spry-sell) (dasatinib) tablets
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: February 2023
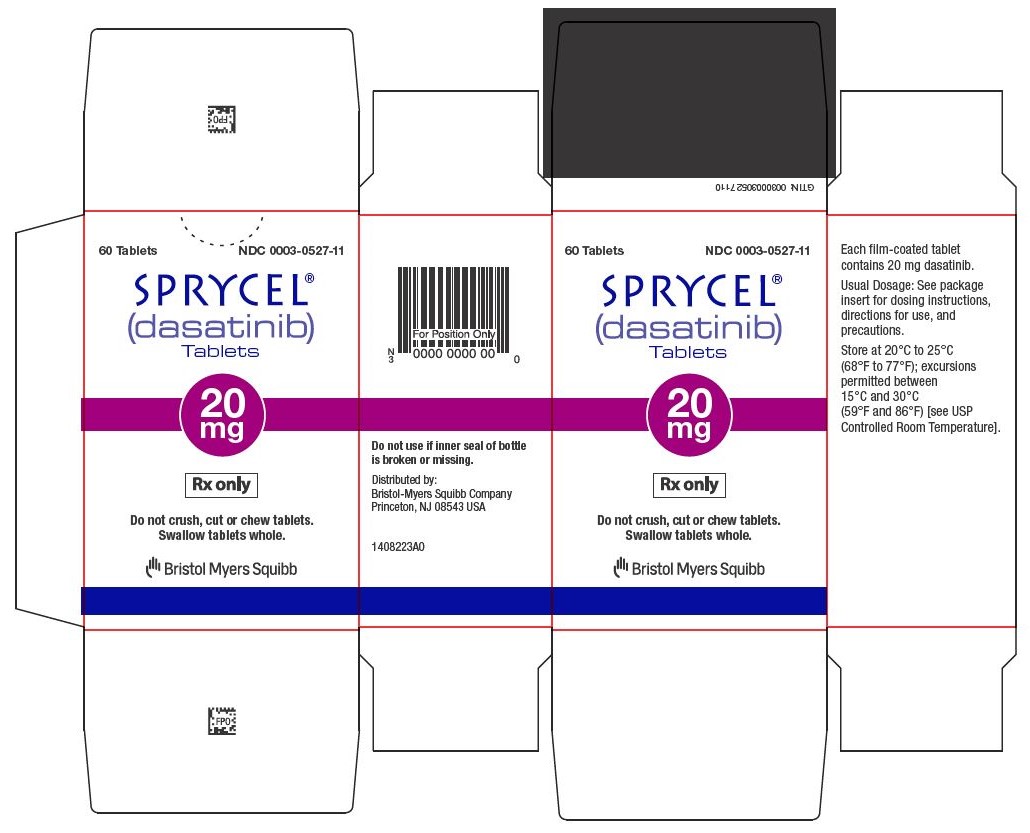
11SPRYCEL 20 mg tablets Representative Packaging
See
60 Tablets NDC 0003-0527-11
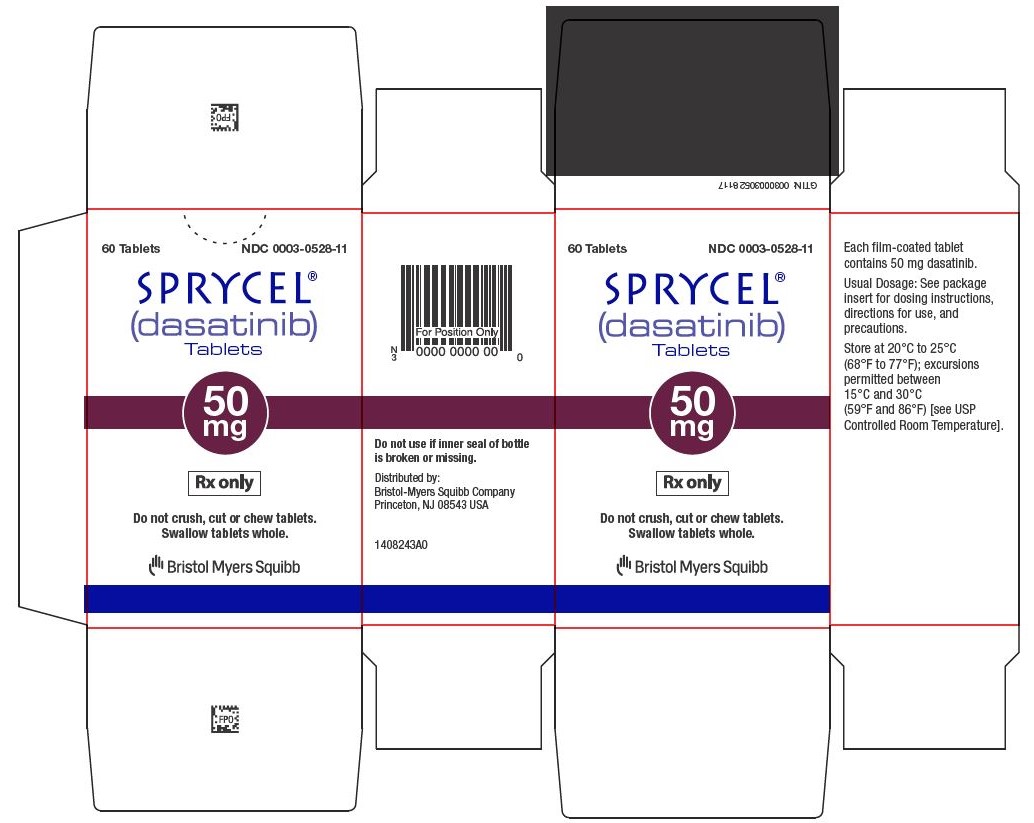
12SPRYCEL 50 mg tablets Representative Packaging
60 Tablets NDC 0003-0528-11
13SPRYCEL 70 mg tablets Representative Packaging
60 Tablets NDC 0003-0524-11
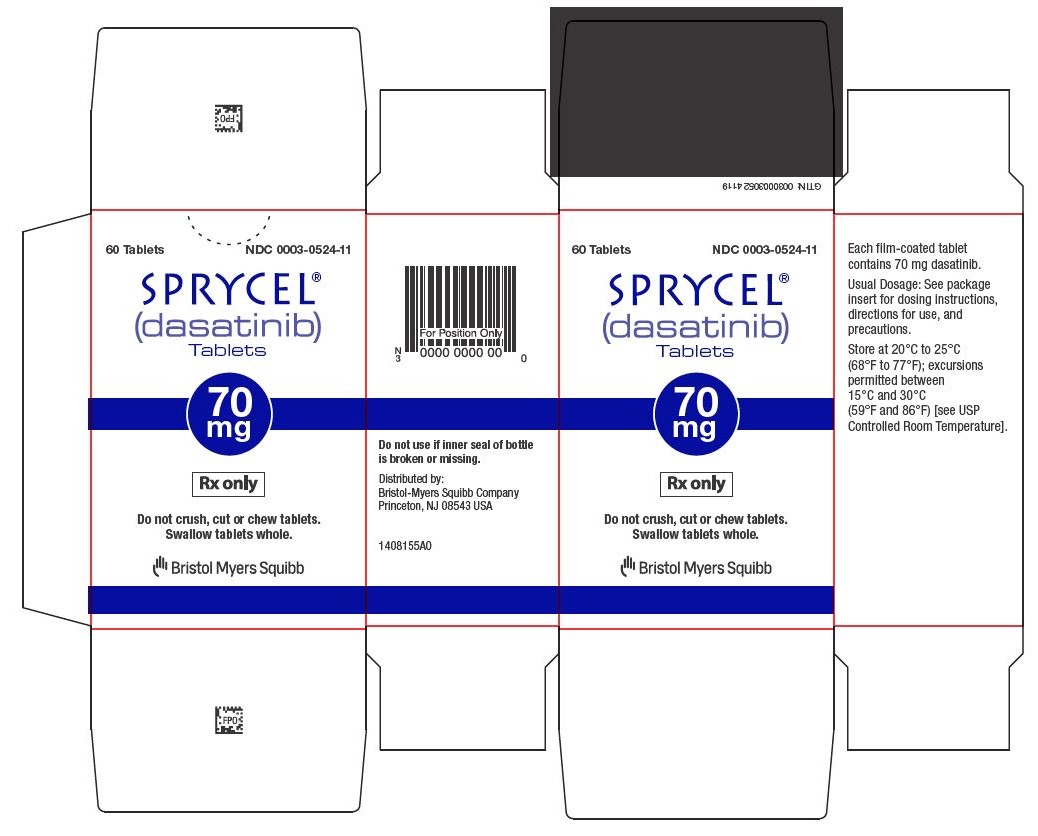
14SPRYCEL 80 mg tablets Representative Packaging
30 Tablets NDC 0003-0855-22
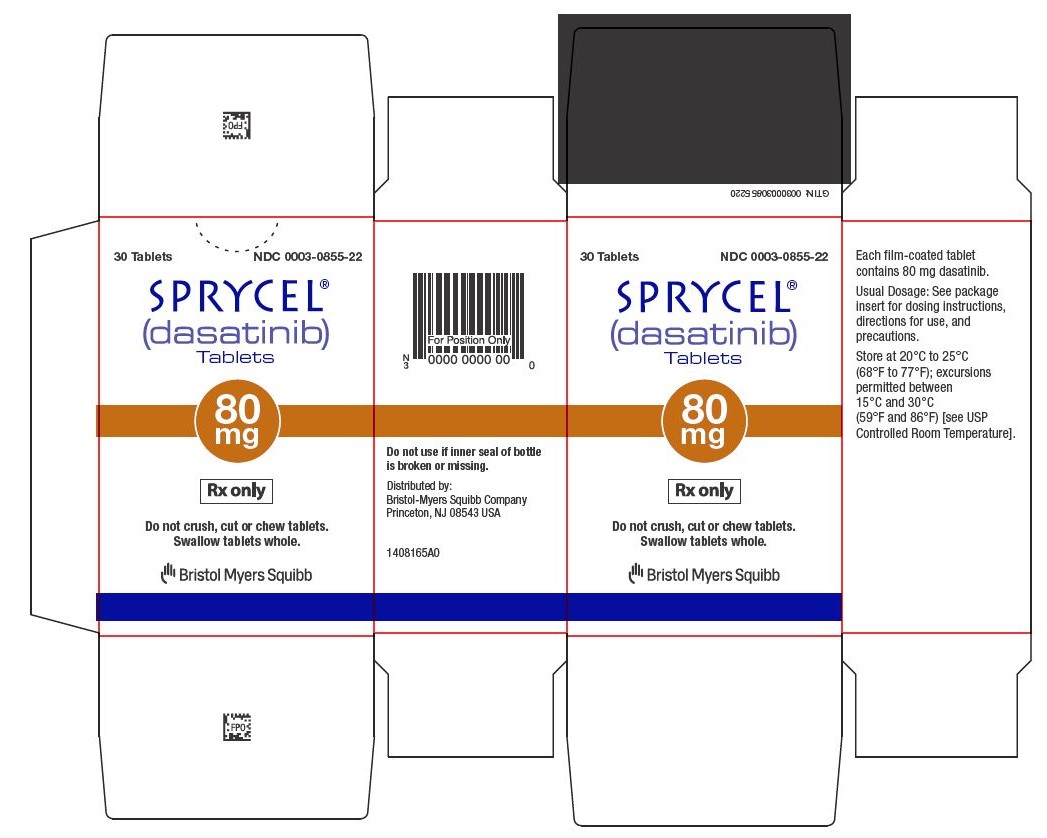
15SPRYCEL 100 mg tablets Representative Packaging
30 Tablets NDC 0003-0852-22
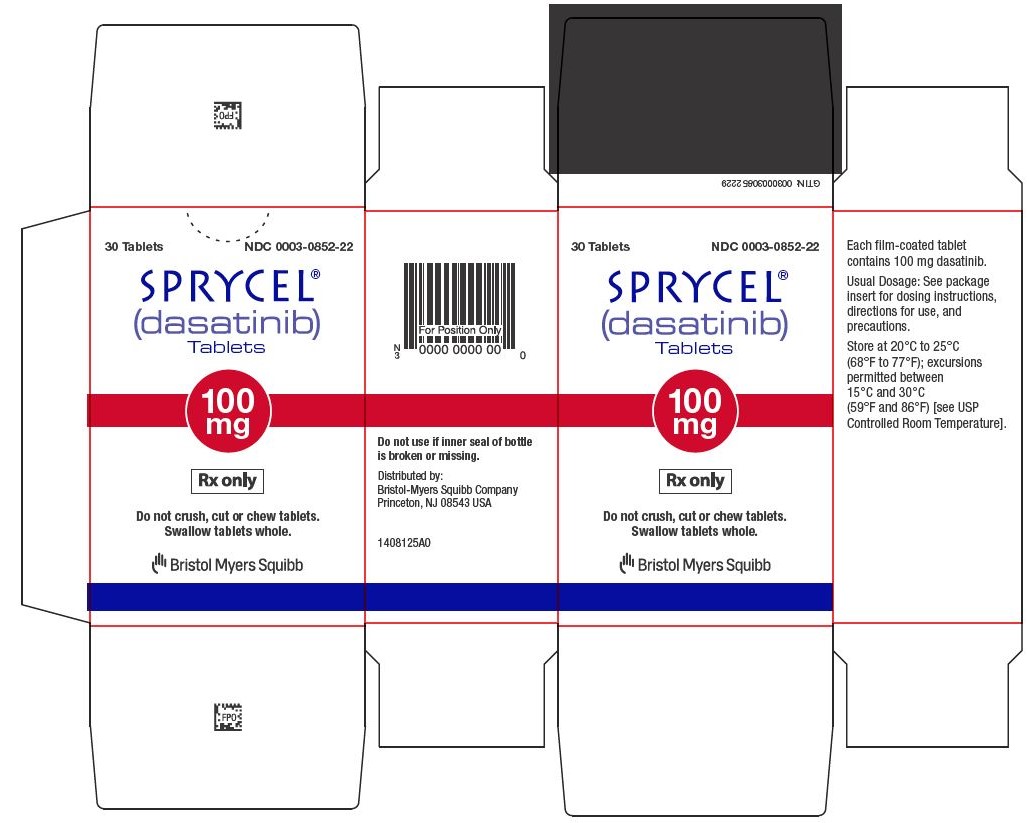
16SPRYCEL 140 mg tablets Representative Packaging
30 Tablets NDC 0003-0857-22