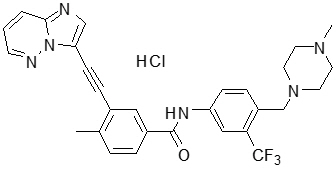
Iclusig
What is Iclusig (Ponatinib)?
Top Global Experts
Related Clinical Trials
Summary: This phase III trial compares the effect of usual treatment of chemotherapy and steroids and a tyrosine kinase inhibitor (TKI) to the same treatment plus blinatumomab. Blinatumomab is a Bi-specific T-Cell Engager ('BiTE') that may interfere with the ability of cancer cells to grow and spread. The information gained from this study may help researchers determine if combination therapy with steroids...
Summary: The purpose of this study is to learn more about LP-118 (an experimental drug) and its side effects and decide on acceptable doses. The purpose of this study is to determine if LP-118 can be given safely with another medicine called ponatinib, that is FDA-approved for the treatment of acute lymphoblastic leukemia.
Summary: The objective of this Phase II study is to assess the potential of asciminib in managing CML-CP or CML-AP in patient carrying the T315I mutation. The presence of this mutation introduces treatment difficulties due to the limited available options. The study seeks to collect additional data on the effectiveness and safety of asciminib for these patients. By determining the drug's capacity to manage...
Related Latest Advances
Brand Information
- Newly diagnosed Ph+ ALL in combination with chemotherapy.
- As monotherapy in Ph+ ALL for whom no other kinase inhibitors are indicated or T315I-positive Ph+ ALL.
- Chronic phase (CP) CML with resistance or intolerance to at least two prior kinase inhibitors.
- Accelerated phase (AP) or blast phase (BP) CML for whom no other kinase inhibitors are indicated.
- T315I-positive CML (chronic phase, accelerated phase, or blast phase).
- 10 mg of ponatinib: Oval, white to off-white, biconvex, debossed "NZ" on one side and plain on the other side
- 15 mg of ponatinib: Round, white, biconvex, debossed "A5" on one side and plain on the other side
- 30 mg of ponatinib: Round, white, biconvex, debossed "C7" on one side and plain on the other side
- 45 mg of ponatinib: Round, white, biconvex, debossed "AP4" on one side and plain on the other side
- Arterial Occlusive Events
- Venous Thromboembolic Events
- Heart Failure
- Hepatotoxicity
- Hypertension
- Pancreatitis
- Neuropathy
- Ocular Toxicity
- Hemorrhage
- Fluid Retention
- Cardiac Arrhythmias
- Myelosuppression
- Tumor Lysis Syndrome
- Reversible Posterior Leukoencephalopathy Syndrome
- Impaired Wound Healing and Gastrointestinal Perforation