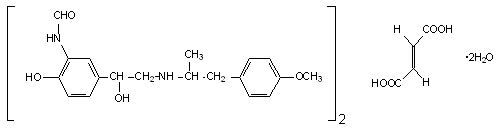
Formoterol
What is Perforomist (Formoterol)?
For many people living with chronic obstructive pulmonary disease (COPD), each breath can feel like a struggle. Shortness of breath, fatigue, and persistent coughing can make daily activities from walking to speaking feel exhausting. Perforomist (formoterol) is designed to help ease that burden, giving patients steadier breathing and better control over their symptoms.
Perforomist is a long-acting beta₂-adrenergic agonist (LABA) used for the maintenance treatment of COPD, including chronic bronchitis and emphysema. It is not a rescue inhaler but a controller medication that works continuously to help the lungs stay open and functioning more efficiently. Approved by the U.S. Food and Drug Administration (FDA), Perforomist offers sustained relief from COPD symptoms and helps improve lung function and overall quality of life when used as prescribed.
What does Perforomist do?
Perforomist helps manage chronic obstructive pulmonary disease (COPD), a long-term condition that makes it hard to breathe because of airway inflammation and obstruction. In COPD, the airways become narrow and clogged with mucus, leading to difficulty exhaling air from the lungs.
Perforomist is used regularly to prevent symptoms, not for sudden breathing problems. By helping airways stay open for up to 12 hours, it allows oxygen to move more freely through the lungs, reducing breathlessness and improving endurance.
In clinical studies, patients who used formoterol experienced significant improvement in lung capacity (measured by FEV₁) and reported fewer COPD flare-ups compared to placebo groups (NIH, 2024). Many patients find that regular use of Perforomist helps them perform daily activities with less fatigue and shortness of breath, contributing to a better quality of life.
How does Perforomist work?
Perforomist contains formoterol fumarate, a long-acting bronchodilator. It works by targeting beta₂-adrenergic receptors in the smooth muscles that line the airways of the lungs.
When activated, these receptors cause the muscles to relax, which widens (dilates) the airways and allows air to pass through more easily. Unlike short-acting inhalers that provide immediate but brief relief, formoterol starts working within minutes and continues to keep airways open for about 12 hours.
This mechanism helps prevent the daily cycle of airway tightening that leads to breathlessness, coughing, and wheezing. By maintaining open airways around the clock, Perforomist supports steady, easier breathing and reduces the risk of exacerbations or hospitalizations.
Clinically, this long-term bronchodilation is essential for COPD management because it helps the lungs work more efficiently, preserves oxygen exchange, and reduces strain on the heart and respiratory muscles.
Perforomist side effects
Most patients tolerate Perforomist well, but like all medications, it can cause side effects. These are usually mild and manageable with proper monitoring.
Common side effects may include:
- Tremors or shakiness
- Headache
- Nervousness or restlessness
- Muscle cramps
- Throat irritation or cough
- Nausea or upset stomach
Less common but serious side effects:
- Chest pain or fast, irregular heartbeat
- Severe dizziness or fainting
- Worsening shortness of breath immediately after use
- Low blood potassium (which can cause muscle weakness or irregular heartbeat)
- Signs of allergic reaction such as rash, itching, or swelling of the face or throat
Because Perforomist is a LABA, it should not be used alone to treat asthma, as this has been linked to an increased risk of asthma-related complications. However, it is safe and FDA-approved for COPD when used as directed.
People with heart disease, high blood pressure, diabetes, thyroid disorders, or seizure disorders should discuss these conditions with their healthcare provider before using the medication.
Seek emergency medical care if you experience chest pain, severe palpitations, or trouble breathing after using the medication. Regular follow-ups with a doctor help ensure that side effects are caught early and treatment remains effective.
Perforomist dosage
Perforomist is an inhalation solution delivered via a nebulizer, ideal for patients who struggle with traditional inhalers. It’s typically used twice daily, 12 hours apart, for consistent relief, and patients should not exceed this frequency. Perforomist is not a rescue medication for sudden breathing problems; a separate short-acting bronchodilator is prescribed for acute symptoms.
Doctors advise regular lung function, heart rate, and blood potassium checks for safe treatment, especially for older adults or those with cardiovascular issues. Mild liver or kidney impairment usually doesn’t require dosage adjustments, but a healthcare provider should always review individual responses.
Does Perforomist have a generic version?
Yes. Formoterol fumarate inhalation solution, the generic version of Perforomist, is FDA-approved and available in the United States. It contains the same active ingredient, dosage strength, and formulation as Perforomist, providing the same effectiveness and safety profile.
Generic formoterol is a safe, FDA-approved, and affordable alternative that improves access to long-term therapy. Other combination inhalers containing formoterol, like Symbicort and Dulera, are distinct medications and should be used only as prescribed.
Conclusion
Perforomist (formoterol) is a proven, long-acting bronchodilator that helps people with COPD breathe more comfortably and live more actively. By relaxing airway muscles and improving airflow, it provides sustained symptom control and reduces the frequency of flare-ups that can interrupt daily life.
While Perforomist is not a quick-relief medication, its consistent, twice-daily use can make a significant difference in long-term respiratory stability and overall well-being. Most patients tolerate it well, and regular monitoring ensures that any side effects or complications are managed promptly.
With guidance from a healthcare professional, Perforomist can be a safe, effective cornerstone of COPD treatment, helping patients breathe easier, move with confidence, and enjoy a fuller life.
References
- U.S. Food and Drug Administration (FDA). (2024). Perforomist (formoterol fumarate) inhalation solution – prescribing information. Retrieved from https://www.accessdata.fda.gov
- Mayo Clinic. (2024). Formoterol (inhalation route): Drug information. Retrieved from https://www.mayoclinic.org
- MedlinePlus. (2024). Formoterol inhalation: Uses, side effects, and precautions. National Library of Medicine. Retrieved from https://medlineplus.gov
- National Institutes of Health (NIH). (2024). Long-acting beta-agonists in COPD management. Retrieved from https://www.nih.gov
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: This study will investigate the effect of Budesonide, Glycopyrronium, and Formoterol Fumarate (BGF) metered dose inhaler (MDI) compared with Placebo MDI, and Budesonide and Formoterol Fumarate (BFF) MDI on isotime inspiratory capacity (IC) and exercise endurance time.
Summary: This is a parallel-group, Phase 2, randomized, double-blind, placebo-controlled, 2-arm study for the treatment of asthma. The purpose of this study is to assess the efficacy, safety, and tolerability of add-on therapy with SC lunsekimig compared with placebo in male and female participants (aged 18 to 80 years, inclusive) with asthma, who are not currently eligible for biologic treatments. Study d...
Summary: This study will evaluate the effect of triple ICS/LAMA/LABA therapy with BGF MDI 320/14.4/9.6 μg on cardiopulmonary outcomes relative to LAMA/LABA therapy with GFF MDI 14.4/9.6 μg in a population with COPD and elevated cardiopulmonary risk.
Related Latest Advances
Brand Information
- PERFOROMIST Inhalation Solution should only be administered via a standard jet nebulizer connected to an air compressor with an adequate airflow and equipped with a facemask or mouthpiece.
- Vial should always be stored in the foil pouch, and only removed IMMEDIATELY before use.
- Do not take by mouth.
- Contents of any partially used container should be discarded.
- Discard the container and top after use.
- Keep out of the reach of children
FORMIS:R3
- Remove vial from the foil pouch.
- Twist the cap completely off the vial and squeeze all the medicine into the nebulizer medicine cup (reservoir) (Figure 1).
- Connect the nebulizer reservoir to the mouthpiece or facemask (Figure 2).
- Connect the nebulizer to the compressor.
- Sit in a comfortable, upright position. Place the mouthpiece in your mouth (Figure 3) or put on the facemask (Figure 4); and turn on the compressor.
- Breathe as calmly, deeply and evenly as possible through your mouth until no more mist is formed in the nebulizer reservoir. The average nebulization time is 9 minutes. At this point, the treatment is finished.
- Discard the PERFOROMIST Inhalation Solution container and top after use.
- Clean the nebulizer (see manufacturer’s instructions).
(formoterol fumarate) INHALATION SOLUTION
20 mcg/2 mL vial