Brand Name
Naratriptan
View Brand InformationFDA approval date: July 07, 2010
Classification: Serotonin-1b and Serotonin-1d Receptor Agonist
Form: Tablet
What is Naratriptan?
Naratriptan is indicated for the acute treatment of migraine attacks with or without aura in adults. Limitations of Use: Use only if a clear diagnosis of migraine has been established. If a patient has no response to the first migraine attack treated with naratriptan, reconsider the diagnosis of migraine before naratriptan is administered to treat any subsequent attacks., Naratriptan is not indicated for the prevention of migraine attacks., Safety and effectiveness of naratriptan have not been established for cluster headache. Naratriptan is a serotonin receptor agonist indicated for the acute treatment of migraine with or without aura in adults. Limitations of Use:, Use only if a clear diagnosis of migraine has been established. , Not indicated for the prophylactic therapy of migraine attacks. , Not indicated for the treatment of cluster headache.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Naratriptan (Naratriptan)
1INDICATIONS AND USAGE
Naratriptan is indicated for the acute treatment of migraine attacks with or without aura in adults.
Limitations of Use:
- Use only if a clear diagnosis of migraine has been established. If a patient has no response to the first migraine attack treated with naratriptan, reconsider the diagnosis of migraine before naratriptan is administered to treat any subsequent attacks.
- Naratriptan is not indicated for the prevention of migraine attacks.
- Safety and effectiveness of naratriptan have not been established for cluster headache.
2DOSAGE FORMS AND STRENGTHS
1 mg white to off-white round, biconvex tablet debossed with “54” on one side and “227” on the other side.
2.5 mg white to off-white round, biconvex tablet debossed with “54 351” on one side and plain on the other side.
3CONTRAINDICATIONS
Naratriptan is contraindicated in patients with:
- Ischemic coronary artery disease (CAD) (angina pectoris, history of myocardial infarction, or documented silent ischemia) or coronary artery vasospasm, including Prinzmetal’s angina
- Wolff-Parkinson-White syndrome or arrhythmias associated with other cardiac accessory conduction pathway disorders
- History of stroke or transient ischemic attack (TIA) or history of hemiplegic or basilar migraine because such patients are at a higher risk of stroke
- Peripheral vascular disease
- Ischemic bowel disease
- Uncontrolled hypertension
- Recent use (i.e., within 24 hours) of another 5-HT
- Hypersensitivity to naratriptan (angioedema and anaphylaxis seen)
- Severe renal or hepatic impairment
4ADVERSE REACTIONS
The following adverse reactions are discussed in more detail in other sections of the prescribing information:
- Myocardial ischemia, myocardial infarction, and Prinzmetal’s angina
- Arrhythmias
- Chest, throat, neck, and/or jaw pain/tightness/pressure
- Cerebrovascular events
- Other vasospasm reactions
- Medication overuse headache
- Serotonin syndrome
- Increase in blood pressure
- Hypersensitivity reactions
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In a long-term open-label trial where patients were allowed to treat multiple migraine attacks for up to 1 year, 15 patients (3.6%) discontinued treatment due to adverse reactions.
In controlled clinical trials, the most common adverse reactions were paresthesias, dizziness, drowsiness, malaise/fatigue, and throat/neck symptoms, which occurred at a rate of 2% and at least 2 times placebo rate.
Table 1 lists the adverse reactions that occurred in 5 placebo-controlled clinical trials of approximately 1,752 exposures to placebo and naratriptan in adult patients with migraine. Only reactions that occurred at a frequency of 2% or more in groups treated with naratriptan 2.5 mg and that occurred at a frequency greater than the placebo group in the 5 pooled trials are included in Table 1.
The incidence of adverse reactions in controlled clinical trials was not affected by age or weight of the patients, duration of headache prior to treatment, presence of aura, use of prophylactic medications, or tobacco use. There were insufficient data to assess the impact of race on the incidence of adverse reactions.
5OVERDOSAGE
Adverse reactions observed after overdoses of up to 25 mg included increases in blood pressure resulting in lightheadedness, neck tension, tiredness, and loss of coordination. Also, ischemic ECG changes likely due to coronary artery vasospasm have been reported.
The elimination half-life of naratriptan is about 6 hours
6DESCRIPTION
Naratriptan Tablets, USP contains naratriptan hydrochloride, USP, a selective 5-HT
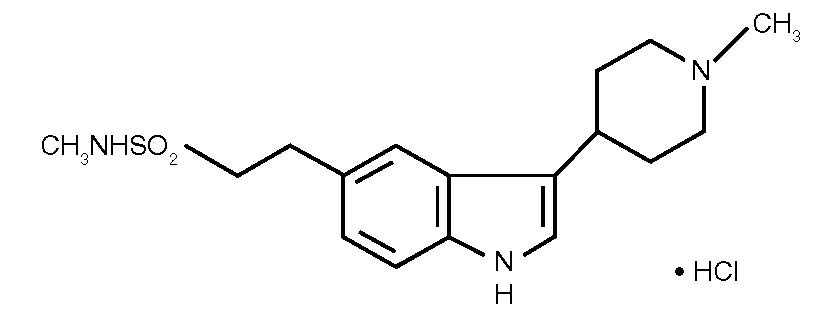
The empirical formula is C
Each naratriptan tablet for oral administration contains 1.11 or 2.78 mg of naratriptan hydrochloride, USP, equivalent to 1 or 2.5 mg of naratriptan, respectively. Each tablet also contains the inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, lactose (anhydrous), magnesium stearate, and microcrystalline cellulose.
7CLINICAL STUDIES
The efficacy of naratriptan in the acute treatment of migraine headaches was evaluated in 3 randomized, double-blind, placebo-controlled trials in adult patients (Trials 1, 2, 3). These trials enrolled adult patients who were predominantly female (86%) and Caucasian (96%) with a mean age of 41 years (range: 18 to 65 years). In all studies, patients were instructed to treat at least 1 moderate to severe headache. Headache response, defined as a reduction in headache severity from moderate or severe pain to mild or no pain, was assessed up to 4 hours after dosing. Associated symptoms such as nausea, vomiting, photophobia, and phonophobia were also assessed. Maintenance of response was assessed for up to 24 hours postdose. A second dose of naratriptan or other rescue medication to treat migraines was allowed 4 to 24 hours after the initial treatment for recurrent headache.
In all 3 trials, the percentage of patients achieving headache response 4 hours after treatment, the primary outcome measure, was significantly greater among patients receiving naratriptan compared with those who received placebo. In all trials, response to 2.5 mg was numerically greater than response to 1 mg and in the largest of the 3 trials, there was a statistically significant greater percentage of patients with headache response at 4 hours in the 2.5-mg group compared with the 1-mg group. The results are summarized in Table 2.
The estimated probability of achieving an initial headache response in adults over the 4 hours following treatment in pooled Trials 1, 2, and 3 is depicted in Figure 1.
Figure : Estimated Probability of Achieving Initial Headache Response Within 4 Hours in Pooled Trials 1, 2, and 3
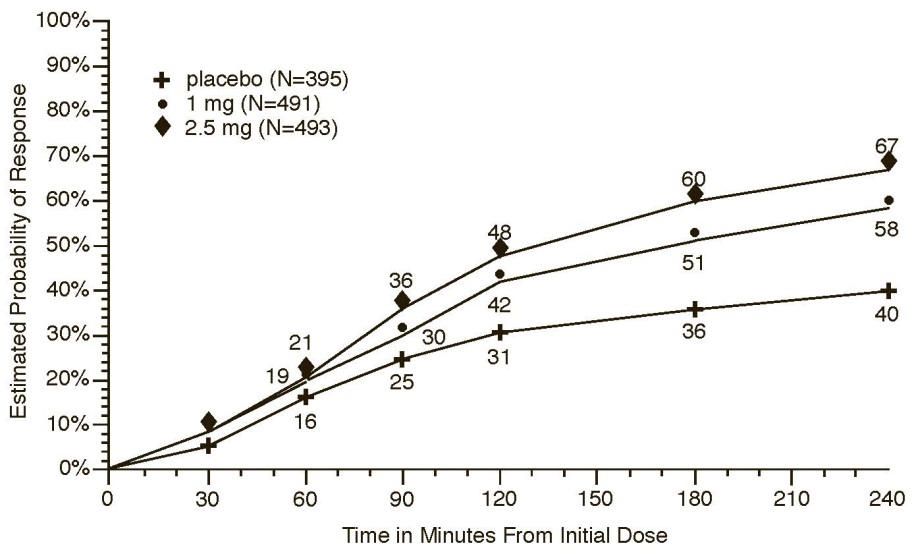
- a The figure shows the probability over time of obtaining headache response (reduction in headache severity from moderate or severe pain to no or mild pain) following treatment with naratriptan. In this Kaplan-Meier plot, patients not achieving response within 240 minutes were censored at 240 minutes.
For patients with migraine-associated nausea, photophobia, and phonophobia at baseline, there was a lower incidence of these symptoms 4 hours following administration of 1-mg and 2.5-mg naratriptan compared with placebo.
Four to 24 hours following the initial dose of study treatment, patients were allowed to use additional treatment for pain relief in the form of a second dose of study treatment or other rescue medication. The estimated probability of patients taking a second dose or other rescue medication to treat migraine over the 24 hours following the initial dose of study treatment is summarized in Figure 2.
Figure : Estimated Probability of Patients Taking a Second Dose of Naratriptan Tablets or Other Medication to Treat Migraine Over the 24 Hours Following the Initial Dose of Study Treatment in Pooled Trials 1, 2, and 3
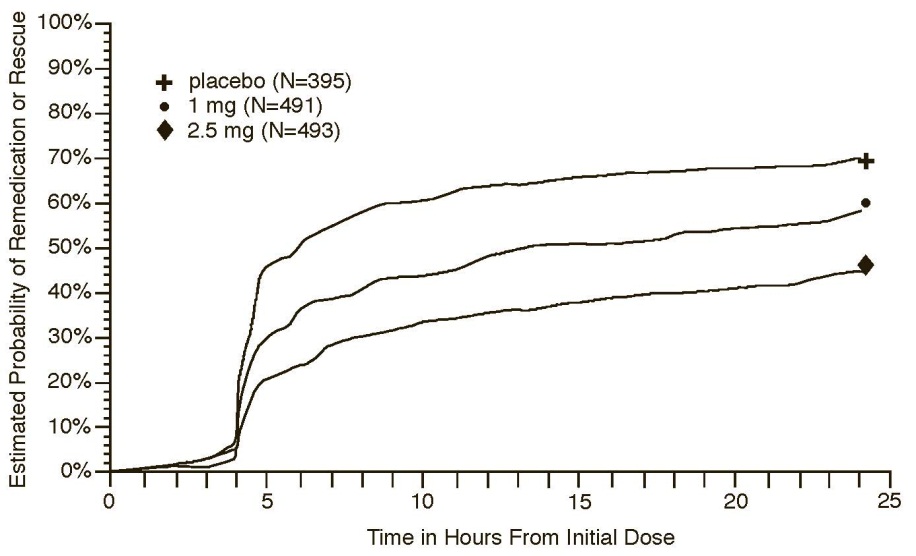
- a Kaplan-Meier plot based on data obtained in the 3 controlled clinical trials (Trials 1, 2, and 3) providing evidence of efficacy with patients not using additional treatments censored at 24 hours. The plot also includes patients who had no response to the initial dose. Remedication was discouraged prior to 4 hours postdose.
There is no evidence that doses of 5 mg provided a greater effect than 2.5 mg. There was no evidence to suggest that treatment with naratriptan was associated with an increase in the severity or frequency of migraine attacks. The efficacy of naratriptan was unaffected by presence of aura; gender, age, or weight of the subject; oral contraceptive use; or concomitant use of common migraine prophylactic drugs (e.g., beta-blockers, calcium channel blockers, tricyclic antidepressants). There was insufficient data to assess the impact of race on efficacy.
8HOW SUPPLIED/STORAGE AND HANDLING
Naratriptan Tablets, USP
1 mg tablets are supplied as white to off-white round, biconvex tablets debossed with “54” on one side and “227” on the other side.
NDC 0054-0278-03: Bottle of 9 Tablets
NDC 0054-0278-25: Bottle of 100 Tablets
2.5 mg tablets are supplied as white to off-white round, biconvex tablets debossed with “54 351” on one side and plain on the other side.
NDC 0054-0279-03: Bottle of 9 Tablets
NDC 0054-0279-25: Bottle of 100 Tablets
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
9PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Risk of Myocardial Ischemia and/or Infarction, Prinzmetal’s Angina, Other Vasospasm-Related Events, Arrhythmias, and Cerebrovascular Events:
Inform patients that naratriptan may cause serious cardiovascular side effects such as myocardial infarction or stroke. Although serious cardiovascular events can occur without warning symptoms, patients should be alert for the signs and symptoms of chest pain, shortness of breath, irregular heartbeat, significant rise in blood pressure, weakness, and slurring of speech and should ask for medical advice if any indicative sign or symptoms are observed. Apprise patients of the importance of this follow-up
Anaphylactic Reactions:
Inform patients that anaphylactic reactions have occurred in patients receiving naratriptan. Such reactions can be life threatening or fatal. In general, anaphylactic reactions to drugs are more likely to occur in individuals with a history of sensitivity to multiple allergens
Concomitant Use with Other Triptans or Ergot Medications:
Inform patients that use of naratriptan within 24 hours of another triptan or an ergot-type medication (including dihydroergotamine or methysergide) is contraindicated
Serotonin Syndrome:
Caution patients about the risk of serotonin syndrome with the use of naratriptan or other triptans, particularly during combined use with SSRIs, SNRIs, TCAs, and MAO inhibitors
Medication Overuse Headache:
Inform patients that use of acute migraine drugs for 10 or more days per month may lead to an exacerbation of headache and encourage patients to record headache frequency and drug use (e.g., by keeping a headache diary)
Pregnancy:
Advise patients to notify their healthcare provider if they become pregnant during treatment or intend to become pregnant
Lactation:
Advise patients to notify their healthcare provider if they are breastfeeding or plan to breastfeed
Ability to Perform Complex Tasks:
Treatment with naratriptan may cause somnolence and dizziness; instruct patients to evaluate their ability to perform complex tasks after administration of naratriptan.
Distributed by:
Revised March 2023
10PATIENT INFORMATION
Naratriptan Tablets, USP
(nar” a trip’ tan)
Rx Only
Read this Patient Information before you start taking naratriptan tablets and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or treatment.
What is the most important information I should know about naratriptan?
Naratriptan can cause serious side effects, including:
Heart attack and other heart problems. Heart problems may lead to death.
Stop taking naratriptan and get emergency medical help right away if you have any of the following symptoms of a heart attack:
- discomfort in the center of your chest that lasts for more than a few minutes, or that goes away and comes back
- severe tightness, pain, pressure, or heaviness in your chest, throat, neck, or jaw
- pain or discomfort in your arms, back, neck, jaw, or stomach
- shortness of breath with or without chest discomfort
- breaking out in a cold sweat
- nausea or vomiting
- feeling lightheaded
Naratriptan is not for people with risk factors for heart disease unless a heart exam is done and shows no problem. You have a higher risk for heart disease if you:
- have high blood pressure
- have high cholesterol levels
- smoke
- are overweight
- have diabetes
- have a family history of heart disease
What is naratriptan?
Naratriptan is a prescription medicine used to treat acute migraine headaches with or without aura in adults who have been diagnosed with migraine headaches.
Naratriptan is not used to prevent or decrease the number of migraine headaches you have.
Naratriptan is not used to treat other types of headaches such as hemiplegic migraines (that make you unable to move on one side of your body) or basilar migraines (rare form of migraine with aura).
It is not known if naratriptan is safe and effective to treat cluster headaches.
It is not known if naratriptan is safe and effective in children younger than 18 years of age.
Who should not take naratriptan?
Do not take naratriptan if you have:
- heart problems or a history of heart problems
- narrowing of blood vessels to your legs, arms, stomach, or kidney (peripheral vascular disease)
- uncontrolled high blood pressure
- severe kidney problems
- severe liver problems
- hemiplegic migraines or basilar migraines. If you are not sure if you have these types of migraines, ask your healthcare provider.
- had a stroke, transient ischemic attacks (TIAs), or problems with your blood circulation
- taken any of the following medicines in the last 24 hours:
Ask your healthcare provider if you are not sure if your medicine is listed above.
- an allergy to naratriptan or any of the ingredients in naratriptan tablets. See the end of this leaflet for a complete list of ingredients in naratriptan tablets.
What should I tell my healthcare provider before taking naratriptan?
Before you take naratriptan, tell your healthcare provider about all of your medical conditions, including if you:
- have high blood pressure
- have high cholesterol
- have diabetes
- smoke
- are overweight
- have heart problems or family history of heart problems or stroke
- have kidney problems
- have liver problems
- are not using effective birth control
- are pregnant or plan to become pregnant
- are breastfeeding or plan to breastfeed. It is not known if naratriptan passes into your breast milk. Talk with your healthcare provider about the best way to feed your baby if you take naratriptan.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Using naratriptan with certain other medicines can affect each other, causing serious side effects.
Especially tell your healthcare provider if you take anti-depressant medicines called:
- selective serotonin reuptake inhibitors (SSRIs)
- serotonin norepinephrine reuptake inhibitors (SNRIs)
- tricyclic antidepressants (TCAs)
- monoamine oxidase inhibitors (MAOIs)
Ask your healthcare provider or pharmacist for a list of these medicines if you are not sure.
Know the medicines you take. Keep a list of them to show your healthcare provider or pharmacist when you get a new medicine.
How should I take naratriptan?
- Certain people should take their first dose of naratriptan in their healthcare provider’s office or in another medical setting. Ask your healthcare provider if you should take your first dose in a medical setting.
- Take naratriptan exactly as your healthcare provider tells you to take it.
- Your healthcare provider may change your dose. Do not change your dose without first talking with your healthcare provider.
- Take naratriptan with water or other liquids.
- If you do not get any relief after your first naratriptan tablet, do not take a second tablet without first talking with your healthcare provider.
- If your headache comes back or you only get some relief from your headache, you can take a second tablet 4 hours after the first tablet.
- Do not take more than a total of 5 mg of naratriptan in a 24-hour period.
- Some people who take too many naratriptan tablets may have worse headaches (medication overuse headache). If your headaches get worse, your healthcare provider may decide to stop your treatment with naratriptan.
- If you take too much naratriptan, call your healthcare provider or go to the nearest hospital emergency room right away.
- You should write down when you have headaches and when you take naratriptan so you can talk with your healthcare provider about how naratriptan is working for you.
What should I avoid while taking naratriptan?
Naratriptan can cause dizziness, weakness, or drowsiness. If you have these symptoms, do not drive a car, use machinery, or do anything where you need to be alert.
What are the possible side effects of naratriptan?
Naratriptan may cause serious side effects. See “What is the most important information I should know about naratriptan?”
These serious side effects include:
- changes in color or sensation in your fingers and toes (Raynaud’s syndrome)
- stomach and intestinal problems (gastrointestinal and colonic ischemic events). Symptoms of gastrointestinal and colonic ischemic events include:
- problems with blood circulation to your legs and feet (peripheral vascular ischemia). Symptoms of peripheral vascular ischemia include:
- medication overuse headaches. Some people who use too many naratriptan tablets may have worse headaches (medication overuse headache). If your headaches get worse, your healthcare provider may decide to stop your treatment with naratriptan.
- serotonin syndrome. Serotonin syndrome is a rare but serious problem that can happen in people using naratriptan, especially if naratriptan is used with antidepressant medicines called SSRIs, SNRIs, TCAs, or MAOIs. Call your healthcare provider right away if you have any of the following symptoms of serotonin syndrome:
The most common side effects of naratriptan include:
- tingling or numbness in your fingers or toes
- dizziness
- warm, hot, burning feeling to your face (flushing)
- discomfort or stiffness in your neck
- feeling weak, drowsy, or tired
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of naratriptan. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store naratriptan?
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Keep naratriptan and all medicines out of the reach of children.
General information about the safe and effective use of naratriptan.
Medicines are sometimes prescribed for purposes other than those listed in Patient Information leaflets. Do not use naratriptan for a condition for which it was not prescribed. Do not give naratriptan to other people, even if they have the same symptoms you have. It may harm them.
This Patient Information leaflet summarizes the most important information about naratriptan. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about naratriptan that is written for healthcare professionals.
For more information call 1-800-962-8364.
What are the ingredients in Naratriptan Tablets, USP?
Active ingredient: naratriptan hydrochloride, USP
Inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, lactose (anhydrous), magnesium stearate and microcrystalline cellulose.
This Patient Information has been approved by the U.S. Food and Drug Administration.
The brands listed are registered trademarks of their respective owners.
Distributed by:
Revised March 2023
11PACKAGE/LABEL PRINCIPAL DISPLAY PANEL

12PACKAGE/LABEL PRINCIPAL DISPLAY PANEL

