Brand Name
Cidofovir
View Brand InformationFDA approval date: August 06, 2012
Classification: Cytomegalovirus Nucleoside Analog DNA Polymerase Inhibitor
Form: Injection
What is Cidofovir?
Cidofovir is indicated for the treatment of CMV retinitis in patients with acquired immunodeficiency syndrome . THE SAFETY AND EFFICACY OF CIDOFOVIR INJECTION HAVE NOT BEEN ESTABLISHED FOR TREATMENT OF OTHER CMV INFECTIONS , CONGENITAL OR NEONATAL CMV DISEASE, OR CMV DISEASE IN NON-HIV-INFECTED INDIVIDUALS.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
An Open-label, Single-arm Study to Evaluate the Safety, Tolerability, Efficacy and Pharmacokinetics of Maribavir in Chinese Transplant Recipients With Cytomegalovirus (CMV) Infections That Are Refractory or Resistant to Treatment With Ganciclovir, Valganciclovir, Cidofovir or Foscarnet
Summary: The main aim of this study is to learn how safe maribavir is in Chinese adults who have undergone hematopoietic stem cell or organ transplantation and have a cytomegalovirus (CMV) infection and how well they tolerate treatment with maribavir. Other aims are to see how effective maribavir is in treating CMV infection and getting rid of the symptoms, the recurrence rate of CMV infection after treatm...
A Phase IIa, Open-label, Multiple Ascending Dose Confirmation Study of the Safety and Tolerability of Intravenous Administration of Brincidofovir in Subjects With Adenovirus Infection or Cytomegalovirus Infection
Summary: The purpose of this study is to determine the safety and tolerability of intravenous (IV) brincidofovir (BCV; SyB V-1901) 0.2 mg/kg, 0.3 mg/kg or 0.4 mg/kg dosed twice weekly (BIW) or 0.4 mg/kg dosed once weekly (QW) for 4 weeks in subjects with AdV, and IV BCV in subjects with CMV
Related Latest Advances
Brand Information
CIDOFOVIR (Cidofovir Anhydrous)
WARNING
RENAL IMPAIRMENT IS THE MAJOR TOXICITY OF CIDOFOVIR INJECTION. CASES OF ACUTE RENAL FAILURE RESULTING IN DIALYSIS AND/OR CONTRIBUTING TO DEATH HAVE OCCURRED WITH AS FEW AS ONE OR TWO DOSES OF CIDOFOVIR INJECTION. TO REDUCE POSSIBLE NEPHROTOXICITY, INTRAVENOUS PREHYDRATION WITH NORMAL SALINE AND ADMINISTRATION OF PROBENECID MUST BE USED WITH EACH CIDOFOVIR INJECTION INFUSION. RENAL FUNCTION (SERUM CREATININE AND URINE PROTEIN) MUST BE MONITORED WITHIN 48 HOURS PRIOR TO EACH DOSE OF CIDOFOVIR INJECTION AND THE DOSE OF CIDOFOVIR INJECTION MODIFIED FOR CHANGES IN RENAL FUNCTION AS APPROPRIATE (SEE
NEUTROPENIA HAS BEEN OBSERVED IN ASSOCIATION WITH CIDOFOVIR INJECTION TREATMENT. THEREFORE, NEUTROPHIL COUNTS SHOULD BE MONITORED DURING CIDOFOVIR INJECTION THERAPY.
CIDOFOVIR INJECTION IS INDICATED ONLY FOR THE TREATMENT OF CMV RETINITIS IN PATIENTS WITH ACQUIRED IMMUNODEFICIENCY SYNDROME.
IN ANIMAL STUDIES CIDOFOVIR WAS CARCINOGENIC, TERATOGENIC AND CAUSED HYPOSPERMIA (SEE
1DESCRIPTION
The chemical name of cidofovir is 1-[(
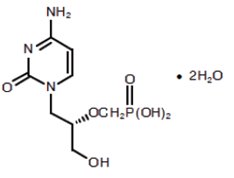
Cidofovir, USP is a white crystalline powder with an aqueous solubility of ≥ 170 mg/mL at pH 6 to 8 and a log P (octanol/aqueous buffer, pH 7.1) value of -3.3.
Cidofovir injection, USP is a sterile, hypertonic aqueous solution for intravenous infusion only. The solution is clear and colorless. It is supplied in clear glass vials, each containing 375 mg of anhydrous cidofovir in 5 mL aqueous solution at a concentration of 75 mg/mL. The formulation is pH-adjusted to 7.4 with sodium hydroxide and/or hydrochloric acid and contains no preservatives. The appropriate volume of cidofovir injection must be removed from the single-dose vial and diluted prior to administration (see
2INDICATIONS AND USAGE
Cidofovir is indicated for the treatment of CMV retinitis in patients with acquired immunodeficiency syndrome (AIDS). THE SAFETY AND EFFICACY OF CIDOFOVIR INJECTION HAVE NOT BEEN ESTABLISHED FOR TREATMENT OF OTHER CMV INFECTIONS (SUCH AS PNEUMONITIS OR GASTROENTERITIS), CONGENITAL OR NEONATAL CMV DISEASE, OR CMV DISEASE IN NON-HIV-INFECTED INDIVIDUALS.
3DESCRIPTION OF CLINICAL TRIALS
Three phase II/III controlled trials of cidofovir injection have been conducted in HIV-infected patients with CMV retinitis.
3.1Delayed Versus Immediate Therapy (Study 105)
In stage 1 of this open-label trial, conducted by the Studies of the Ocular Complications of AIDS (SOCA) Clinical Research Group, 29 previously untreated patients with peripheral CMV retinitis were randomized to either immediate treatment with cidofovir injection (5 mg/kg once a week for 2 weeks, then 3 mg/kg every other week) or to have cidofovir injection delayed until progression of CMV retinitis
3.2Delayed Versus Immediate Therapy (Study 106)
In an open-label trial, 48 previously untreated patients with peripheral CMV retinitis were randomized to either immediate treatment with cidofovir injection (5 mg/kg once a week for 2 weeks, then 5 mg/kg every other week), or to have cidofovir injection delayed until progression of CMV retinitis
3.3Dose-Response Study of Cidofovir Injection (Study 107)
In an open-label trial, 100 patients with relapsing CMV retinitis were randomized to receive 5 mg/kg once a week for 2 weeks and then either 5 mg/kg (n = 49) or 3 mg/kg (n = 51) every other week. Enrolled patients had been diagnosed with CMV retinitis an average of 390 days prior to randomization and had received a median of 3.8 prior courses of systemic CMV therapy. Eighty-four of the 100 patients were considered evaluable for progression by serial retinal photographs (43 randomized to 5 mg/kg and 41 randomized to 3 mg/kg). Twenty-six and 21 patients discontinued therapy due to either an adverse event, intercurrent illness, excluded medication, or withdrawn consent in the 5 mg/kg and 3 mg/kg groups, respectively. Thirty-eight of the 100 randomized patients had progressed according to masked assessment of serial retinal photographs (13 randomized to 5 mg/kg and 25 randomized to 3 mg/kg). Using retinal photographs, the median (95% CI) times to retinitis progression for the 5 mg/kg and 3 mg/kg groups were 115 days (70, not reached) and 49 days (35, 52), respectively. This difference was statistically significant. Similar to Study 106, the median time to retinitis progression for the 5 mg/kg group was difficult to precisely estimate due to the limited number of patients remaining on treatment over time (4 of the 49 patients in the 5 mg/kg group were treated for 115 days or longer). Median (95% CI) times to the alternative endpoint of retinitis progression or study drug discontinuation were 49 days (38, 63) and 35 days (27, 39) for the 5 mg/kg and 3 mg/kg groups, respectively. This difference was statistically significant.
4CONTRAINDICATIONS
Initiation of therapy with cidofovir injection is contraindicated in patients with a serum creatinine > 1.5 mg/dL, a calculated creatinine clearance ≤ 55 mL/min, or a urine protein ≥ 100 mg/dL (equivalent to ≥ 2+ proteinuria).
Cidofovir injection is contraindicated in patients receiving agents with nephrotoxic potential. Such agents must be discontinued at least seven days prior to starting therapy with cidofovir injection.
Cidofovir injection is contraindicated in patients with hypersensitivity to cidofovir.
Cidofovir injection is contraindicated in patients with a history of clinically severe hypersensitivity to probenecid or other sulfa-containing medications.
Direct intraocular injection of cidofovir injection is contraindicated; direct injection of cidofovir has been associated with iritis, ocular hypotony, and permanent impairment of vision.
5ADVERSE REACTIONS
- Nephrotoxicity: Renal toxicity, as manifested by ≥ 2+ proteinuria, serum creatinine elevations of ≥ 0.4 mg/dL, or decreased creatinine clearance ≤ 55 mL/min, occurred in 79 of 135 (59%) patients receiving cidofovir injection at a maintenance dose of 5 mg/kg every other week. Maintenance dose reductions from 5 mg/kg to 3 mg/kg due to proteinuria or serum creatinine elevations were made in 12 of 41 (29%) patients who had not received prior therapy for CMV retinitis (Study 106) and in 19 of 74 (26%) patients who had received prior therapy for CMV retinitis (Study 107). Prior foscarnet use has been associated with an increased risk of nephrotoxicity; therefore, such patients must be monitored closely (see CONTRAINDICATIONS, WARNINGS, DOSAGE AND ADMINISTRATION).
- Neutropenia: In clinical trials, at the 5 mg/kg maintenance dose, a decrease in absolute neutrophil count to ≤ 500 cells/mm3 occurred in 24% of patients. Granulocyte colony stimulating factor (GCSF) was used in 39% of patients.
- Decreased Intraocular Pressure/Ocular Hypotony: Among the subset of patients monitored for intraocular pressure changes, a ≥ 50% decrease from baseline intraocular pressure was reported in 17 of 70 (24%) patients at the 5 mg/kg maintenance dose. Severe hypotony (intraocular pressure of 0 to 1 mm Hg) has been reported in three patients. Risk of ocular hypotony may be increased in patients with preexisting diabetes mellitus.
- Anterior Uveitis/Iritis: Uveitis or iritis has been reported in clinical trials and during post-marketing in patients receiving cidofovir injection therapy. Uveitis or iritis was reported in 15 of 135 (11%) patients receiving 5 mg/kg maintenance dosing. Treatment with topical corticosteroids with or without topical cycloplegic agents may be considered. Patients should be monitored for signs and symptoms of uveitis/iritis during cidofovir injection therapy.
- Metabolic Acidosis: A diagnosis of Fanconi's syndrome, as manifested by multiple abnormalities of proximal renal tubular function, was reported in 1% of patients. Decreases in serum bicarbonate to ≤ 16 mEq/L occurred in 16% of cidofovir-treated patients. Cases of metabolic acidosis in association with liver dysfunction and pancreatitis resulting in death have been reported in patients receiving cidofovir injection.
In clinical trials, cidofovir injection was withdrawn due to adverse events in 39% of patients treated with 5 mg/kg every other week as maintenance therapy.
The incidence of adverse reactions reported as serious in three controlled clinical studies in patients with CMV retinitis, regardless of presumed relationship to drug, is listed in Table 4.
The most frequently reported adverse events regardless of relationship to study drugs (cidofovir or probenecid) or severity are shown in Table 5.
The following additional list of adverse events/intercurrent illnesses have been observed in clinical studies of cidofovir injection and are listed below regardless of causal relationship to cidofovir injection. Evaluation of these reports was difficult because of the diverse manifestations of the underlying disease and because most patients received numerous concomitant medicines.
Body as a Whole: abdominal pain, accidental injury, AIDS, allergic reaction, back pain, catheter blocked, cellulitis, chest pain, chills and fever, cryptococcosis, cyst, death, face edema, flu-like syndrome, hypothermia, injection site reaction, malaise, mucous membrane disorder, neck pain, overdose, photosensitivity reaction, sarcoma, sepsis
Cardiovascular System: cardiomyopathy, cardiovascular disorder, congestive heart failure, hypertension, hypotension, migraine, pallor, peripheral vascular disorder, phlebitis, postural hypotension, shock, syncope, tachycardia, vascular disorder, edema
Digestive System: cholangitis, colitis, constipation, esophagitis, dyspepsia, dysphagia, fecal incontinence, flatulence, gastritis, gastrointestinal hemorrhage, gingivitis, hepatitis, hepatomegaly, hepatosplenomegaly, jaundice, abnormal liver function, liver damage, liver necrosis, melena, pancreatitis, proctitis, rectal disorder, stomatitis, aphthous stomatitis, tongue discoloration, mouth ulceration, tooth caries
Endocrine System: adrenal cortex insufficiency
Hemic and Lymphatic System: hypochromic anemia, leukocytosis, leukopenia, lymphadenopathy, lymphoma like reaction, pancytopenia, splenic disorder, splenomegaly, thrombocytopenia, thrombocytopenic purpura
Metabolic and Nutritional System: cachexia, dehydration, edema, hypercalcemia, hyperglycemia, hyperkalemia, hyperlipemia, hypocalcemia, hypoglycemia, hypoglycemic reaction, hypokalemia, hypomagnesemia, hyponatremia, hypophosphatemia, hypoproteinemia, increased alkaline phosphatase, increased BUN, increased lactic dehydrogenase, increased SGOT, increased SGPT, peripheral edema, respiratory alkalosis, thirst, weight loss, weight gain
Musculoskeletal System: arthralgia, arthrosis, bone necrosis, bone pain, joint disorder, leg cramps, myalgia, myasthenia, pathological fracture
Nervous System: abnormal dreams, abnormal gait, acute brain syndrome, agitation, amnesia, anxiety, ataxia, cerebrovascular disorder, confusion, convulsion, delirium, dementia, depression, dizziness, drug dependence, dry mouth, encephalopathy, facial paralysis, hallucinations, hemiplegia, hyperesthesia, hypertonia, hypotony, incoordination, increased libido, insomnia, myoclonus, nervousness, neuropathy, paresthesia, personality disorder, somnolence, speech disorder, tremor, twitching, vasodilatation, vertigo
Respiratory System: asthma, bronchitis, epistaxis, hemoptysis, hiccup, hyperventilation, hypoxia, increased sputum, larynx edema, lung disorder, pharyngitis, pneumothorax, rhinitis, sinusitis
Skin and Appendages: acne, angioedema, dry skin, eczema, exfoliative dermatitis, furunculosis, herpes simplex, nail disorder, pruritus, rash, seborrhea, skin discoloration, skin disorder, skin hypertrophy, skin ulcer, sweating, urticaria
Special Senses: abnormal vision, amblyopia, blindness, cataract, conjunctivitis, corneal lesion, corneal opacity, diplopia, dry eyes, ear disorder, ear pain, eye disorder, eye pain, hyperacusis, iritis, keratitis, miosis, otitis externa, otitis media, refraction disorder, retinal detachment, retinal disorder, taste perversion, tinnitus, uveitis, visual field defect, hearing loss
Urogenital System: decreased creatinine clearance, dysuria, glycosuria, hematuria, kidney stone, mastitis, metorrhagia, nocturia, polyuria, prostatic disorder, toxic nephrophathy, urethritis, urinary casts, urinary incontinence, urinary retention, urinary tract infection
5.1Reporting of Adverse Reactions
To report SUSPECTED ADVERSE REACTIONS, contact Drug Safety at 1-888-875-1671 or FDA Medwatch at 1-800-FDA-1088 or www.fda.gov/medwatch.
6OVERDOSAGE
Two cases of cidofovir overdose have been reported. These patients received single doses of cidofovir injection at 16.3 mg/kg and 17.4 mg/kg, respectively, with concomitant oral probenecid and intravenous hydration. In both cases, the patients were hospitalized and received oral probenecid (one gram 3 times daily) and vigorous intravenous hydration with normal saline for 3 to 5 days. Significant changes in renal function were not observed in either patient.
7DOSAGE AND ADMINISTRATION
CIDOFOVIR INJECTION MUST NOT BE ADMINISTERED BY INTRAOCULAR INJECTION.
7.1Dosage
THE RECOMMENDED DOSAGE, FREQUENCY, OR INFUSION RATE MUST NOT BE EXCEEDED. CIDOFOVIR INJECTION MUST BE DILUTED IN 100 MILLILITERS 0.9% (NORMAL) SALINE PRIOR TO ADMINISTRATION. TO MINIMIZE POTENTIAL NEPHROTOXICITY, PROBENECID AND INTRAVENOUS SALINE PREHYDRATION MUST BE ADMINISTERED WITH EACH CIDOFOVIR INJECTION INFUSION.
7.2Induction Treatment
The recommended induction dose of cidofovir injection for patients with a serum creatinine of ≤ 1.5 mg/dL, a calculated creatinine clearance > 55 mL/min, and a urine protein < 100 mg/dL (equivalent to < 2+ proteinuria) is 5 mg/kg body weight (given as an intravenous infusion at a constant rate over 1 hr) administered once weekly for two consecutive weeks.
Because serum creatinine in patients with advanced AIDS and CMV retinitis may not provide a complete picture of the patient's underlying renal status, it is important to utilize the Cockcroft-Gault formula to more precisely estimate creatinine clearance (Cr
Maintenance Treatment
The recommended maintenance dose of cidofovir injection is 5 mg/kg body weight (given as an intravenous infusion at a constant rate over one hr), administered once every 2 weeks.
7.3Probenecid
Probenecid must be administered orally with each cidofovir injection dose. Two grams must be administered 3 hr prior to the cidofovir injection dose and one gram administered at 2 and again at 8 hr after completion of the one hr cidofovir injection infusion (for a total of 4 grams).
Ingestion of food prior to each dose of probenecid may reduce drug-related nausea and vomiting. Administration of an antiemetic may reduce the potential for nausea associated with probenecid ingestion. In patients who develop allergic or hypersensitivity symptoms to probenecid, the use of an appropriate prophylactic or therapeutic antihistamine and/or acetaminophen should be considered (see
7.4Hydration
Patients must receive at least one liter of 0.9% (normal) saline solution intravenously with each infusion of cidofovir injection. The saline solution should be infused over a 1 to 2 hr period immediately before the cidofovir injection infusion. Patients who can tolerate the additional fluid load should receive a second liter. If administered, the second liter of saline should be initiated either at the start of the cidofovir injection infusion or immediately afterwards, and infused over a 1 to 3 hr period.
7.5Method of Preparation and Administration
Inspect vials visually for particulate matter and discoloration prior to administration. If particulate matter or discoloration is observed, the vial should not be used. With a syringe, extract the appropriate volume of cidofovir injection from the vial and transfer the dose to an infusion bag containing 100 mL 0.9% (normal) saline solution. Infuse the entire volume intravenously into the patient at a constant rate over a one hr period. Use of a standard infusion pump for administration is recommended.
It is recommended that cidofovir injection infusion admixtures be administered within 24 hr of preparation and that refrigerator or freezer storage not be used to extend this 24 hr limit.
If admixtures are not intended for immediate use, they may be stored under refrigeration (2°C to 8°C) for no more than 24 hr. Refrigerated admixtures should be allowed to equilibrate to room temperature prior to use.
The chemical stability of cidofovir injection admixtures was demonstrated in polyvinyl chloride composition and ethylene/propylene copolymer composition commercial infusion bags, and in glass bottles.
Cidofovir injection is supplied in single-dose vials. Partially used vials should be discarded (see
Compatibility with Ringer's solution, Lactated Ringer's solution or bacteriostatic infusion fluids has not been evaluated.
7.6Handling and Disposal
Due to the mutagenic properties of cidofovir, adequate precautions including the use of appropriate safety equipment are recommended for the preparation, administration, and disposal of cidofovir injection. The National Institutes of Health presently recommends that such agents be prepared in a Class II laminar flow biological safety cabinet and that personnel preparing drugs of this class wear surgical gloves and a closed front surgical-type gown with knit cuffs. If cidofovir injection contacts the skin, wash membranes and flush thoroughly with water. Excess cidofovir injection and all other materials used in the admixture preparation and administration should be placed in a leak-proof, puncture-proof container. The recommended method of disposal is high temperature incineration.
7.7Patient Monitoring
Serum creatinine and urine protein must be monitored within 48 hours prior to each dose. White blood cell counts with differential should be monitored prior to each dose. In patients with proteinuria, intravenous hydration should be administered and the test repeated. Intraocular pressure, visual acuity and ocular symptoms should be monitored periodically.
8HOW SUPPLIED
Cidofovir Injection USP, 75 mg per mL, for intravenous infusion, is available in:
NDC 67457-210-05
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Preservative Free
Sterile, Nonpyrogenic
Discard unused portion.
9REFERENCES
- Ho HT, Woods KL, Bronson JJ, De Boeck H, Martin JC and Hitchcock MJM. Intracellular Metabolism of the Antiherpesvirus Agent (S)-1-[3-hydroxy-2-(phosphonylmethoxy) propyl]cytosine.
- Cherrington JM, Allen SJW, McKee BH, and Chen MS. Kinetic Analysis of the Interaction Between the Diphosphate of (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine, zalcitabineTP, zidovudineTP, and FIAUTP with Human DNA Polymerases b and g.
- Xiong X, Smith JL, Kim C, Huang E, and Chen MS. Kinetic Analysis of the Interaction of Cidofovir Diphosphate with Human Cytomegalovirus DNA Polymerase.
- Cherrington JM, Mulato AS, Fuller MD, Chen MS.
- Stanat SC, Reardon JE, Erice A, Jordan MC, Drew WL, and Biron KK. Ganciclovir-Resistant Cytomegalovirus Clinical Isolates: Mode of Resistance to Ganciclovir.
- Sullivan V, Biron KK, Talarico C, Stanat SC, Davis M, Pozzi M, and Coen DM. A Point Mutation in the Human Cytomegalovirus DNA Polymerase Gene Confers Resistance to Ganciclovir and phosphonylmethoxyalkyl Derivatives.
- Tatarowicz WA, Lurain NS, and Thompson KD. A Ganciclovir-Resistant Clinical Isolate of Human Cytomegalovirus Exhibiting Cross-Resistance to other DNA Polymerase Inhibitors.
- Lurain NS, Thompson KD, Holmes EW, and Read GS. Point Mutations in the DNA Polymerase Gene of Human Cytomegalovirus that Result in Resistance to Antiviral Agents.
- Smith IL, Cherrington JM, Jiles RE, Fuller MD, Freeman WR, Spector SA. High-level Resistance of Cytomegalovirus to Ganciclovir is Associated with Alterations in both the UL97 and DNA Polymerase Genes.
- Sullivan V and Coen DM. Isolation of Foscarnet-Resistant Human Cytomegalovirus Patterns of Resistance and Sensitivity to Other Antiviral Drugs.
- Snoeck R, Andrei G, and De Clercq E. Patterns of Resistance and Sensitivity to Antiviral Compounds of Drug-Resistant Strains of Human Cytomegalovirus Selected
- Baldanti F, Underwood MR, Stanat SC, Biron KK, Chou S, Sarasini A, Silini E, and Gerna G. Single Amino Acid Changes in the DNA Polymerase Confer Foscarnet Resistance and Slow-Growth Phenotype, While Mutations in the UL97-Encoded Phosphotransferase Confer Ganciclovir Resistance in Three Double-Resistant Human Cytomegalovirus Strains Recovered from Patients with AIDS.
- The Studies of Ocular Complications of AIDS Research Group in Collaboration with the AIDS Clinical Trials Group. Cidofovir (HPMPC) for the Treatment of Cytomegalovirus Retinitis in Patients with AIDS: the HPMPC Peripheral Cytomegalovirus Retinitis Trial.
- Lalezari JP, Stagg RJ, Kupperman BD, et al. Intravenous Cidofovir for Peripheral Cytomegalovirus Retinitis in Patients with AIDS. A Randomized, Controlled Trial.
Manufactured for:
Manufactured by:
0782L103
Revised: 4/2021
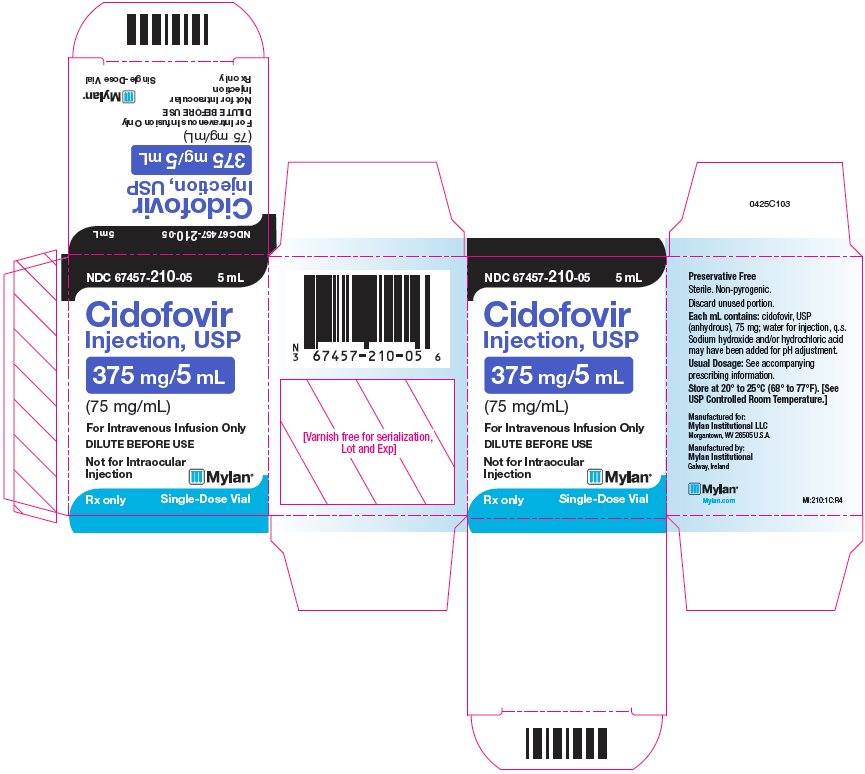
10PRINCIPAL DISPLAY PANEL – 375 mg/5 mL
NDC 67457-210-05 5 mL
Cidofovir
For Intravenous Infusion Only
DILUTE BEFORE USE
Not for Intraocular
Rx only Single-Dose Vial
Preservative Free
Sterile. Non-pyrogenic.
Discard unused portion.
Each mL contains: cidofovir, USP
(anhydrous), 75 mg; water for injection, q.s.
Sodium hydroxide and/or hydrochloric acid
may have been added for pH adjustment.
(anhydrous), 75 mg; water for injection, q.s.
Sodium hydroxide and/or hydrochloric acid
may have been added for pH adjustment.
Usual Dosage: See accompanying
prescribing information.
prescribing information.
Store at 20° to 25°C (68° to 77°F). [See
Manufactured for:
Manufactured by:
Mylan.com
MI:210:1C:R4