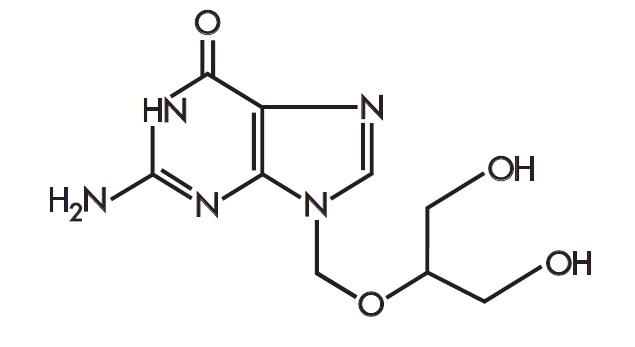
Zirgan
What is Zirgan (Ganciclovir)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: This is a Phase 1 single-arm open-label study of letermovir in neonates with symptomatic congenital Cytomegalovirus (CMV) disease. There will be two groups enrolled. Group 1 will be comprised of 4 subjects. Following documentation study inclusion and signing of informed consent, Group 1 subjects will receive one dose of oral letermovir (Study Day 0), using the dose bands. A full pharmacokinetics (...
Summary: the existing anti-CMV drugs mainly include valganciclovir, ganciclovir and foscarnet sodium, all of which act on DNA polymerase (pUL54), making them prone to cross resistance. DNA synthesis in normal cell is also catalyzed by DNA polymerase, which can also inhibit normal cell production, especially in metabolically active bone marrow cells, leading to bone marrow suppression. In addition, these dr...
Summary: This study is being conducted at seven major children's hospitals in Australia and New Zealand to test a new approach for treating a virus, called cytomegalovirus in children with weakened immune systems. The researchers want to find out if using a web app to customise the dose of a medication called ganciclovir is better at clearing the virus over a six-week period compared to the standard method...
Related Latest Advances
Brand Information
Bridgewater, NJ 08807 USA
Manufactured by:
Bausch & Lomb Incorporated
Tampa, FL 33637 USA




