Micardis
What is Micardis (Telmisartan)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: Parkinson's disease (PD) is currently the fastest-growing neurological condition globally. It is projected to affect 172,000 people in the UK by 2030,with the current annual cost to the country being \ £3.6 billion. The disease progressively impairs physical abilities, leading to increased disability, falls, and difficulties with speech, swallowing, mood, thinking, and memory. While existing treat...
Summary: This is a research study to test the safety and effectiveness of using the drug alpelisib together with chemotherapy (nab-paclitaxel) and a drug called L-NMMA in patients with HER2 negative metastatic or locally advanced metaplastic breast cancer, who have not responded to previous treatments. Participants in this study in addition to the standard care chemotherapy will also receive the drug alpel...
Summary: \[Primary Objective\] To demonstrate the superiority of the change in mean sitting systolic blood pressure (MSSBP) and hemoglobin A1c (HbA1c)on week 12 of the combination therapy of THP-00101 (dapagliflozin 10 mg) and THP-00102 (telmisartan 80 mg) compared to THP-00101 or THP-00102 monotherapy among subjects with type 2 diabetes mellitus accompanied by essential hypertension. \[Secondary Objective...
Related Latest Advances
Brand Information
- When pregnancy is detected, discontinue MICARDIS HCT as soon as possible
- Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus
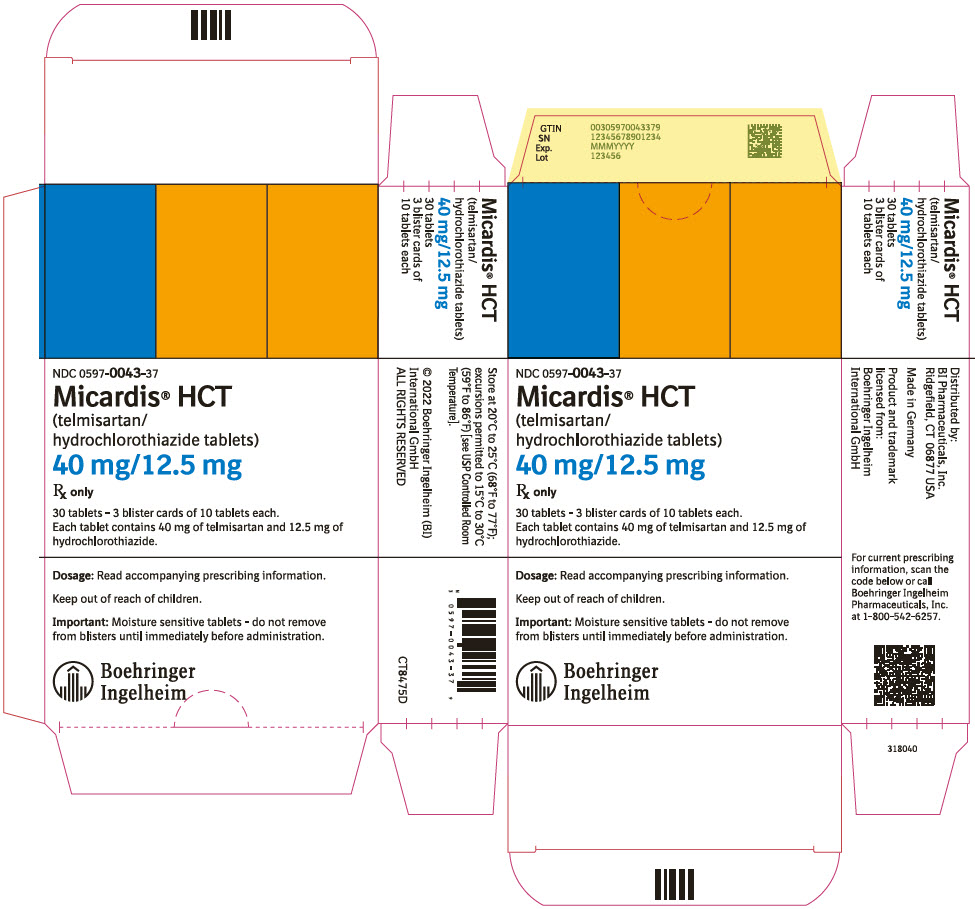
- 40 mg/12.5 mg, red and white tablets marked with the Boehringer Ingelheim logo and "H4"
- 80 mg/12.5 mg, red and white tablets marked with the Boehringer Ingelheim logo and "H8"
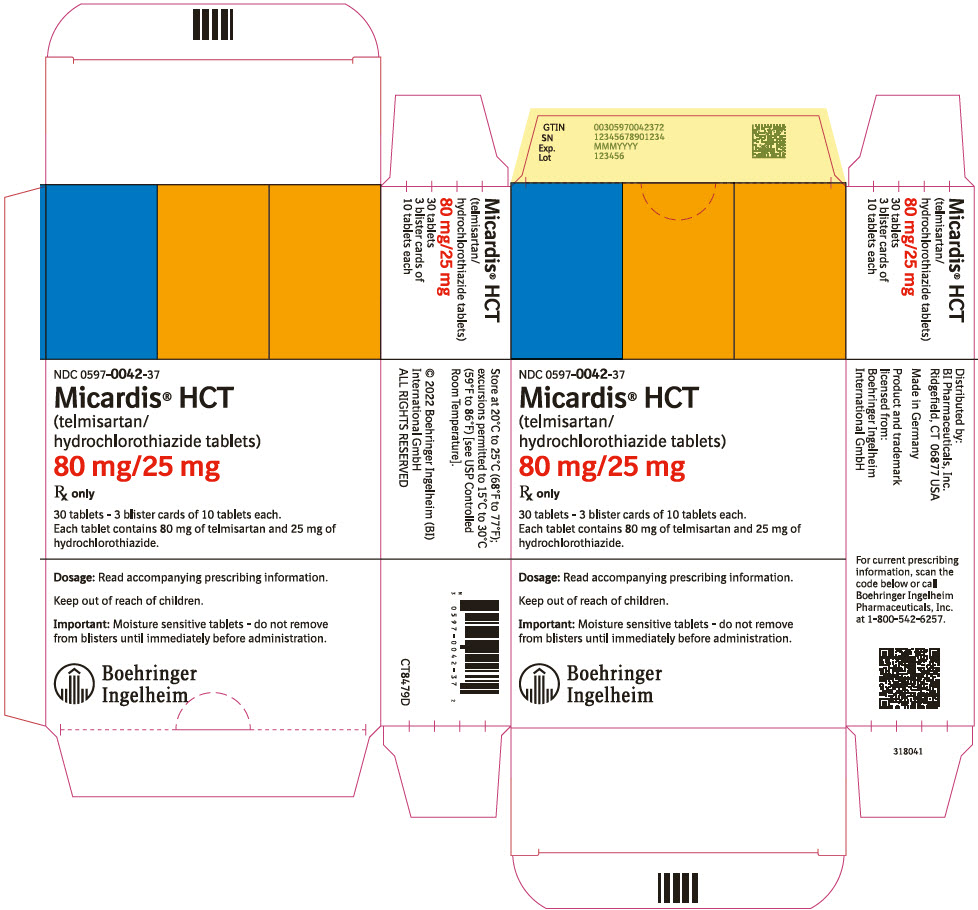
- 80 mg/25 mg, yellow and white tablets marked with the Boehringer Ingelheim logo and "H9"
- In patients who are hypersensitive to any component of this product
- In patients with anuria.
- For co-administration with aliskiren in patients with diabetes
- Hypotension
- Renal Impairment
- Electrolytes and Metabolic Disorders
- 40 mg/12.5 mg tablet: red and white (may contain red specks) marked with the Boehringer Ingelheim company symbol and "H4"; individually blister-sealed in cartons of 30 tablets as 3 × 10 cards (NDC 0597-0043-37)
- 80 mg/12.5 mg tablet: red and white (may contain red specks) marked with the Boehringer Ingelheim company symbol and "H8"; individually blister-sealed in cartons of 30 tablets as 3 × 10 cards (NDC 0597-0044-37)
- 80 mg/25 mg tablet: yellow and white (may contain yellow specks) marked with the Boehringer Ingelheim company symbol and "H9"; individually blister-sealed in cartons of 30 tablets as 3 × 10 cards (NDC 0597-0042-37)
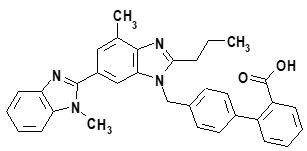
- telmisartan, an angiotensin receptor blocker (ARB)
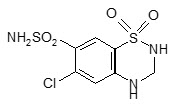
- hydrochlorothiazide, a water pill or diuretic
- have low or no urine output
- are allergic (hypersensitive) to the active ingredients (telmisartan or hydrochlorothiazide) or any of the other ingredients listed at the end of this leaflet
- are pregnant or are planning to become pregnant. See
- are breast-feeding or plan to breast-feed. MICARDIS HCT can pass into your breast milk and may harm your baby. You and your doctor should decide if you will take MICARDIS HCT or breast-feed. You should not do both. Talk with your doctor about the best way to feed your baby if you take MICARDIS HCT tablets.
- have been told that you have abnormal body salt (electrolytes) levels in your blood
- have liver problems
- have asthma or history of asthma
- have lupus
- have diabetes
- have kidney problems
- have any other medical conditions
- aliskiren
- digoxin (Lanoxin
- lithium (Lithobid
- other medicines used to treat your high blood pressure or a heart problem
- water pills (diuretic)
- aspirin or other non-steroidal anti-inflammatory drugs (NSAIDs)
- potassium supplements or a salt substitute containing potassium
- medicine used to treat diabetes, including insulin
- narcotic pain medicines
- sleeping pills
- steroid medicine or Adrenocorticotrophic Hormone (ACTH)
- barbiturates
- certain cholesterol lowering medicines (resins that are used for cholesterol reduction, e.g., cholestyramine and colestipol resins)
- Take MICARDIS HCT tablets exactly as your doctor tells you to take it.
- Your doctor will tell you how much MICARDIS HCT to take and when to take it.
- Do not change your dose unless your doctor tells you to.
- Take MICARDIS HCT once each day.
- Take MICARDIS HCT tablets with or without food.
- If you take too much MICARDIS HCT, call your doctor, or go to the nearest hospital emergency room right away.
- Read the
- Injury or death to your unborn baby. See "
- Low blood pressure (hypotension) is most likely to happen if you also:
- take water pills (diuretics)
- are on a low-salt diet
- get dialysis treatments
- have heart problems
- get sick with vomiting or diarrhea
- do not drink enough fluids
- sweat a lot
- Kidney problems, which may get worse if you already have kidney disease. You may have changes in your kidney test results, and you may need a lower dose of MICARDIS HCT tablets. Call your doctor if you get:
- swelling in your feet, ankles, or hands
- unexplained weight gain
- Liver problems, which may get worse in people who already have liver problems and take MICARDIS HCT.
- Eye problems. One of the medicines in MICARDIS HCT can cause eye problems that may lead to vision loss. Symptoms of eye problems can happen within hours to weeks of starting MICARDIS HCT. Tell your doctor right away if you have:
- decrease in vision
- eye pain
- Allergic reactions. Tell your doctor right away if you get any of these symptoms:
- swelling of the face, tongue, throat
- difficulty breathing
- Worsening of lupus. Tell your doctor if your lupus gets worse or becomes active while taking MICARDIS HCT.
- Change in body salts (electrolytes) level in your blood and fluid problems. Your doctor may do tests to check your blood. Call your doctor right away if you have:
- Skin Cancer. One of the medicines in MICARDIS HCT may increase your risk of getting non-melanoma skin cancer. Protect your skin from the sun and undergo regular skin cancer screening when taking MICARDIS HCT.
- upper respiratory tract infections, including sinus pain/congestion and sore throat
- dizziness
- feeling tired
- flu-like symptoms
- back pain
- diarrhea
- nausea
- Store MICARDIS HCT tablets at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not remove MICARDIS HCT tablets from blisters until right before you take them.

- Tear (You may also use scissors to tear the blister apart)
- Peel (Peel off the paper layer from the aluminum foil)
- Push (Push the tablet through the aluminum foil)