Brand Name
Gliadel
Generic Name
Carmustine
View Brand Information FDA approval date: December 13, 2012
Classification: Alkylating Drug
Form: Kit, Wafer
What is Gliadel (Carmustine)?
Carmustine for injection, USP is indicated as palliative therapy as a single agent or in established combination therapy in the following: - Brain tumors glioblastoma, brainstem glioma, medulloblastoma, astrocytoma, ependymoma, and metastatic brain tumors. - Multiple myeloma in combination with prednisone. - Relapsed or refractory Hodgkin's lymphoma in combination with other approved drugs. - Relapsed or refractory Non-Hodgkin's lymphomas in combination with other approved drugs. Carmustine for injection, USP is a nitrosourea indicated as palliative therapy as a single agent or in established combination therapy with other approved chemotherapeutic agents in the following: Brain tumors glioblastoma, brainstem glioma, medulloblastoma, astrocytoma, ependymoma, and metastatic brain tumors Multiple myeloma-in combination with prednisone Relapsed or refractory Hodgkin's lymphoma in combination with other approved drugs Relapsed or refractory Non-Hodgkin's lymphomas in combination with other approved drugs
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Gliadel (carmustine)
1INDICATIONS AND USAGE
GLIADEL Wafer is indicated for the treatment of patients with:
- newly-diagnosed high-grade glioma as an adjunct to surgery and radiation, and
- recurrent glioblastoma as an adjunct to surgery.
2DOSAGE FORMS AND STRENGTHS
GLIADEL Wafer is an off-white to pale yellow round wafer. Each GLIADEL Wafer contains 7.7 mg of carmustine.
3CONTRAINDICATIONS
None.
4ADVERSE REACTIONS
The following serious adverse reactions are discussed elsewhere in the labeling:
- Seizures
- Intracranial Hypertension
- Impaired Neurosurgical Wound Healing
- Meningitis
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
5DESCRIPTION
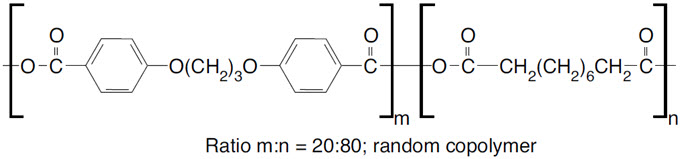
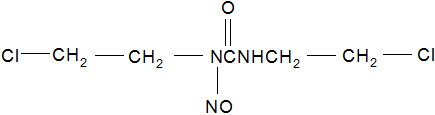
GLIADEL Wafer is an implant for intracranial use, containing carmustine, a nitrosourea alkylating agent, and polifeprosan, a biodegradable copolymer used to control the release of carmustine. It is a sterile, off-white to pale yellow wafer approximately 1.45 cm in diameter and 1 mm thick. Each wafer contains 7.7 mg of carmustine [1, 3-bis (2-chloroethyl)-1-nitrosourea, or BCNU] and 192.3 mg of a biodegradable polyanhydride copolymer. The copolymer, polifeprosan 20, consists of poly [bis (p-carboxyphenoxy)] propane and sebacic acid in a 20:80 molar ratio. Carmustine is homogeneously distributed in the copolymer matrix.
The structural formula for polifeprosan 20 is:
The structural formula for carmustine is:
6REFERENCES
- "OSHA Hazardous Drugs".
7HOW SUPPLIED/STORAGE AND HANDLING
GLIADEL Wafer is supplied in a single dose treatment box containing eight individually pouched wafers. Each wafer contains 7.7 mg of carmustine and is packaged in two aluminum foil laminate pouches. The inner pouch is sterile and is designed to maintain product sterility and protect the product from moisture. The outer pouch is a peelable overwrap. The outside surface of the outer pouch is not sterile.
NDC for single dose treatment box: 24338-050-08
8PRINCIPAL DISPLAY PANEL - 8 Wafer Box
NDC 24338-050-08
GLIADEL
(carmustine implant)
(carmustine implant)
7.7 mg carmustine/wafer
For intracranial use.
Each sterile wafer contains
Contents:
Store at or below -20°C (-4°F).
Rx only
Rx only
Warning:Cytotoxic agent
See package insert for full prescribing information.
Keep out of the reach of children.
See package insert for full prescribing information.
Keep out of the reach of children.
Azurity PHARMACEUTICALS, Inc.