Brand Name
Natacyn
Generic Name
Natamycin
View Brand Information FDA approval date: June 12, 2021
Classification: Polyene Antimicrobial
Form: Suspension
What is Natacyn (Natamycin)?
NATACYN™ 5% is indicated for the treatment of fungal blepharitis, conjunctivitis, and keratitis caused by susceptible organisms including Fusarium solani keratitis. As in other forms of suppurative keratitis, initial and sustained therapy of fungal keratitis should be determined by the clinical diagnosis, laboratory diagnosis by smear and culture of corneal scrapings and drug response. Whenever possible the in vitro activity of natamycin against the responsible fungus should be determined. The effectiveness of natamycin as a single agent in fungal endophthalmitis has not been established.
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
Fungal Ulcer Treatment Augmented With Natamycin and Cyclosporine A
Summary: The goal of this clinical trial is to learn if adjunctive topical Cyclosporine A eye drops combined with standard of care topical Natamycin treatment improves vision outcomes in patients with fungal keratitis.
Related Latest Advances
Brand Information
NATACYN (natamycin)
1DESCRIPTION:
NATACYN™ (natamycin ophthalmic suspension) 5% is a sterile, antifungal drug for topical ophthalmic administration. Each mL of NATACYN™ (natamycin ophthalmic suspension) contains:
The active ingredient is represented by the chemical structure:
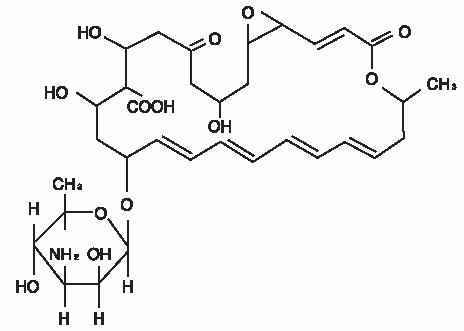
Established Name:Natamycin
Molecular Formula:C 33H 47NO 13
Molecular Weight:665.73 g/mol
Chemical Name:Stereoisomer of 22-[(3-amino-3,6-dideoxy- β-D-mannopyranosyl)oxy]-1,3,26- trihydroxy-12- methyl-10-oxo-6,11,28- trioxatricyclo[22.3.1.05,7] octacosa-8,14,16,18,20-pentaene-25- carboxylic acid.
Other:Pimaricin
The pH range is 5.0-7.5.
2CLINICAL PHARMACOLOGY:
Natamycin is a tetraene polyene antibiotic derived from
3INDICATIONS AND USAGE:
NATACYN™ (natamycin ophthalmic suspension) 5% is indicated for the treatment of fungal blepharitis, conjunctivitis, and keratitis caused by susceptible organisms including
4CONTRAINDICATIONS:
NATACYN™ (natamycin ophthalmic suspension) 5% is contraindicated in individuals with a history of hypersensitivity to any of its components.
5PRECAUTIONS: General.
FOR TOPICAL OPHTHALMIC USE ONLY — NOT FOR INJECTION. Failure of improvement of keratitis following 7-10 days of administration of the drug suggests that the infection may be caused by a microorganism not susceptible to natamycin.
Continuation of therapy should be based on clinical re-evaluation and additional laboratory studies.
Adherence of the suspension to areas of epithelial ulceration or retention of the suspension in the fornices occurs regularly. Use only if the container is undamaged.
6Information for Patients:
Do not touch dropper tip to any surface, as this may contaminate the suspension. Patients should be advised not to wear contact lenses if they have signs and symptoms of fungal blepharitis, conjunctivitis, and keratitis.
7Carcinogenesis, Mutagenesis, Impairment of Fertility:
There have been no long term studies done using natamycin in animals to evaluate carcinogenesis, mutagenesis, or impairment of fertility.
8Pregnancy:
Animal reproduction studies have not been conducted with natamycin. It is also not known whether natamycin can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. NATACYN
9Nursing Mothers:
It is not known whether these drugs are excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when natamycin is administered to a nursing woman.
10Pediatric Use:
Safety and effectiveness in pediatric patients have not been established.
11Geriatric Use:
No overall differences in safety or effectiveness have been observed between elderly and younger patients.
12ADVERSE REACTIONS:
The following events have been identified during post-marketing use of NATACYN
13DOSAGE AND ADMINISTRATION:
SHAKE WELL BEFORE USING. The preferred initial dosage in fungal keratitis is one drop of NATACYN™ (natamycin ophthalmic suspension) 5% instilled in the conjunctival sac at hourly or two-hourly intervals. The frequency of application can usually be reduced to one drop 6 to 8 times daily after the first 3 to 4 days. Therapy should generally be continued for 14 to 21 days or until there is resolution of active fungal keratitis. In many cases, it may be helpful to reduce the dosage gradually at 4 to 7 day intervals to assure that the replicating organism has been eliminated. Less frequent initial dosage (4 to 6 daily applications) may be sufficient in fungal blepharitis and conjunctivitis.
14HOW SUPPLIED:
NATACYN™ (natamycin ophthalmic suspension) 5% is a 15 mL fill packaged in a 15 mL amber glass bottle with a black closure. A flint glass dropper with a red plastic closure and a black rubber bulb are packaged separately in a clear plastic blister with Tyvek backing.
15 mL NDC82667-012-05
STORAGE:Store between 2°C to 24°C (36°F-75°F). Do not freeze. Avoid exposure to light and excessive heat.
Rx Only