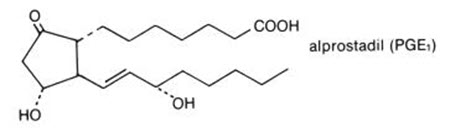
Alprostadil
What is Prostin (Alprostadil)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: Prospective Registry Investigating Maternal, Infant, and Lactation Outcomes in Anifrolumab Users (PRIMULA Lac) is a Post Marketing Requirements (PMR) study designed to fulfill the FDA post-marketing requirements. The study will collect data about the presence of anifrolumab in human breast milk and serum (maternal and infant) among lactating individuals who receive anifrolumab therapeutically.
Summary: This study will investigate the effect of music on postictal agitation when played peri-interventionally for patients undergoing electroconvulsive therapy for severe depression.
Summary: This early phase I trial evaluates the feasibility and impact of a meditation headband system (MUSE-S) for breast cancer survivors. Anxiety and insomnia are among the most common distresses in breast cancer survivors during and after chemotherapy, in part due to the side effects of chemotherapy, fear of cancer coming back, progression of the cancer, and uncertainty of the future. These distresses ...
Related Latest Advances
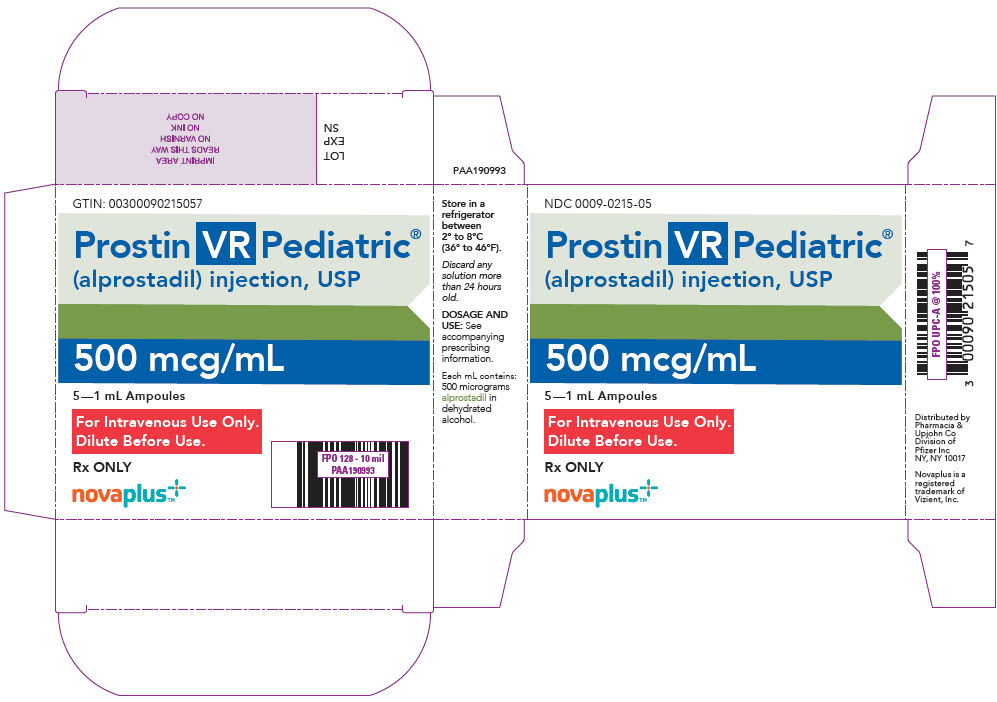
Brand Information