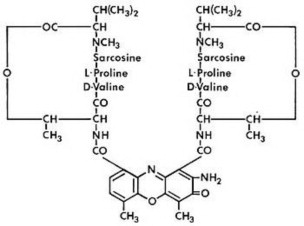
Dactinomycin
View Brand InformationWhat is Dactinomycin?
Approved To Treat
Related Clinical Trials
Summary: This single arm study is designed to demonstrate the feasibility of a radically different approach for an exceptionally high-risk subset of MES with widely metastatic disease (WMES). We incorporate the use of evolutionary principles that apply to species and population dynamics as related to adaptation and extinction to populations of cancer cells that similarly adapt and that we are attempting to...
Summary: This is an open-label, comprehensive, iterative investigation of evaluating the use of induction chemotherapy, high-dose chemotherapy, and focal radiation therapy in children with newly diagnosed Embryonal Tumor With Multilayered Rosettes (ETMR).
Summary: This phase III trial tests how well surgery plus chemotherapy compared to surgery alone works in treating patients with type I pleuropulmonary blastoma (PPB), and tests how well surgery plus standard chemotherapy with the addition of topotecan works compared to surgery plus standard chemotherapy alone in treating patients with type II and III PPB. Historically, most children with type I PPB had su...
Related Latest Advances
Brand Information
- Secondary Malignancy and Leukemia
- Veno-occlusive Disease
- Extravasation
- Myelosuppression
- Immunizations
- Severe Mucocutaneous Reactions
- Renal Toxicity
- Hepatotoxicity
- Potentiation of Radiation Toxicity and Radiation Recall