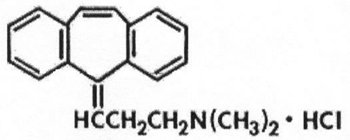
Cyclobenzaprine
What is AMRIX (Cyclobenzaprine)?
Approved To Treat
Related Clinical Trials
Summary: This study will examine the safety and efficacy of TNX-102 SL to reduce ASR symptoms and behavioral changes among patients presenting to the emergency department (ED) after motor vehicle collision (MVC). Specifically, the investigators will perform the Optimizing Acute Stress reaction Interventions with TNX-102 SL (OASIS) Trial, a double-blind placebo-controlled randomized clinical trial (RCT) to ...
Summary: Over half of women in the US who are breastfeeding their infants take prescription drugs. You are being asked to participate in this study because you are breastfeeding your infant and are currently taking, as part of your medical care, at least one of the drugs we are studying. We are interested in studying drugs commonly prescribed to women who are breastfeeding so we can learn more about the am...
Summary: Rationale: Low back pain (LBP), or myofascial pain syndrome (MPS) of the low back, accounts for approximately 2.63 million visits in the United States, or 2.3 percent of annual Emergency Department (ED) visits. An estimated 100 billion dollars per year is lost from LBP. Approximately one-third of this is direct costs. Previous studies have established the safety of trigger point injections (TPI). ...
Related Latest Advances
Brand Information
- AMRIX should be used only for short periods (up to two or three weeks) because adequate evidence of effectiveness for more prolonged use is not available and because muscle spasm associated with acute, painful musculoskeletal conditions is generally of short duration and specific therapy for longer periods is seldom warranted.
- AMRIX has not been found effective in the treatment of spasticity associated with cerebral or spinal cord disease or in children with cerebral palsy.
- It is recommended that doses be taken at approximately the same time each day.
- Use of AMRIX for periods longer than two or three weeks is not recommended
- Sprinkle the contents of the capsule onto a tablespoon of applesauce and consume immediately without chewing.
- Rinse the mouth to ensure all of the contents have been swallowed.
- Discard any unused portion of the AMRIX capsules after the contents have been sprinkled on applesauce.
- 15 mg: Capsules are orange/orange and are embossed in blue ink with “15 mg” on the body, and Cephalon “C” logo, “Cephalon,” and a dashed band on the cap.
- 30 mg: Capsules are blue/red and are embossed in white ink with “30 mg” on the body, and Cephalon “C” logo, “Cephalon,” and a dashed band on the cap.
- Hypersensitivity to any component of this product. Adverse reactions may include anaphylactic reaction, urticaria, facial and/or tongue swelling, or pruritus. Discontinue AMRIX if a hypersensitivity reaction is suspected.
- Concomitant use of monoamine oxidase (MAO) inhibitors or within 14 days after their discontinuation. Hyperpyretic crisis seizures and deaths have occurred in patients receiving cyclobenzaprine (or structurally similar tricyclic antidepressants) concomitantly with MAO inhibitor drugs.
- During the acute recovery phase of myocardial infarction, and in patients with arrhythmias, heart block or conduction disturbances, or congestive heart failure.
- Hyperthyroidism.
- Serotonin Syndrome
- Adverse Cardiovascular Effects [see Warnings and Precautions (
- Instruct patients to swallow AMRIX capsules intact or to sprinkle capsule contents on a tablespoon of applesauce and swallow immediately without chewing.
- Advise patients to stop taking AMRIX and to notify their physician right away if they experience symptoms of an allergic reaction, such as difficulty breathing, hives, swelling of face or tongue, or itching.
- Advise patients that AMRIX should not be taken with MAO inhibitors or within 14 days after their discontinuation.
- Caution patients about the risk of serotonin syndrome with concomitant use of AMRIX and other drugs, such as SSRIs, SNRIs, TCAs, tramadol, bupropion, meperidine, verapamil, or MAO inhibitors. Advise patients of the signs and symptoms of serotonin syndrome
- Advise patients to stop taking AMRIX and to notify their physician right away if they experience arrhythmias or tachycardia.
- Advise patients that AMRIX may enhance the impairment effects of alcohol. These effects may also be seen if AMRIX is taken with other CNS depressants.
- Caution patients about operating an automobile or other hazardous machinery until it is reasonably certain that AMRIX therapy will not adversely affect their ability to engage in such activities.
- Advise patients to take AMRIX at approximately the same time each day.
- Do not chew AMRIX capsules or the granules that are in the capsules.
- The capsule sprinkle method for mixing the contents of AMRIX with applesauce may be used for adults who cannot swallow capsules.
- paper towels
- tablespoon
- applesauce
- cup of water
- Store AMRIX capsules at room temperature between 68°F to 77°F (20°C to 25°C).
60 Capsules

60 Capsules
