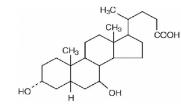
Ursodiol
What is Urso 250 (Ursodiol)?
Approved To Treat
Related Clinical Trials
Summary: This is a prospective, open-label, randomized controlled trial conducted at Lattakia University Hospital (Tishreen University), aiming to evaluate the efficacy of Ursodeoxycholic Acid (UDCA) as an adjuvant to triple phototherapy in the management of indirect hyperbilirubinemia in neonates ≥34 weeks of gestation. Eligible neonates will be randomly assigned to one of two groups: Group A: continuous ...
Summary: The goal of this clinical trial is to investigate the efficacy of ursodeoxycholic acid in treating gastric intestinal metaplasia in Helicobacter pylori-negative adults. It will also learn about the safety of ursodeoxycholic acid. The main questions it aims to answer are: Does ursodeoxycholic acid promote the regression of IM in individuals without Helicobacter pylori infection? What medical proble...
Summary: The objective of this clinical trial is to evaluate the efficacy and safety of CnU capsule 750 mg administration in patients with Cholesterol gallstone (radiolucent gallstones)
Related Latest Advances
Brand Information
- URSO 250: 250 mg tablet
- URSO Forte: 500 mg scored tablet
Allergan USA, Inc.
Madison, NJ 07940
www.allergan.com

NDC 58914-785-10
100 tablets
Rx only
Urso® 250
(Ursodiol Tablets, USP) 250 mg
Allergan™

NDC 58914-790-10
100 tablets
Urso Forte®
(Ursodiol Tablets, USP)
500 mg
Rx only
For the treatment of
Primary Biliary Cirrhosis
Allergan™





