Metoclopramide
What is Reglan (Metoclopramide)?
Approved To Treat
Related Clinical Trials
Summary: The goal of this clinical trial is to evaluate the efficacy and safety of olanzapine plus metoclopramide in preventing opioid-induced nausea and vomiting (OINV) in adult patients with advanced cancer who are initiating strong opioid therapy. The main questions it aims to answer are: (1) Does the combination of olanzapine and metoclopramide reduce the incidence of OINV? (2)What adverse events do pa...
Summary: Metoclopramide is a drug approved by the FDA for gastroesophageal reflux and to relieve symptoms in adults with acute and recurrent diabetic gastroparesis. The objective of this study is to determine whether metoclopramide can improve hypoglycemia awareness and decrease the incidence of hypoglycemia in type 1 diabetes patients with hypoglycemia unawareness.
Summary: The purpose of this study is to evaluate the change in the expression of treatment targets on the surface of tumor cells (Prostate Specific Membrane Antigen (PSMA), Somatostatin Receptor 2 (SSTR2), and Gastrin Releasing Peptide Receptor (GRPR) between the start and after the completion of radioligand therapy (RLT). Study will use radioligand imaging (RLI) to determine predominantly expressed targe...
Related Latest Advances
Brand Information
- Reglan can cause tardive dyskinesia (TD), a serious movement disorder that is often irreversible. There is no known treatment for TD. The risk of developing TD increases with duration of treatment and total cumulative dosage
- Discontinue Reglan in patients who develop signs or symptoms of TD. In some patients, symptoms may lessen or resolve after Reglan is stopped
- Avoid treatment with Reglan for longer than 12 weeks because of the increased risk of developing TD with longer-term use 2.2, 2.3)].
- Treatment for 4 to 12 weeks of symptomatic, documented gastroesophageal reflux in adults who fail to respond to conventional therapy.
- Relief of symptoms in adults with acute and recurrent diabetic gastroparesis.
- 5 mg metoclopramide: green, elliptical-shaped, debossed “REGLAN” over “5” on one side and “ANI” on the opposite side
- 10 mg metoclopramide: white, double edge scored, capsule-shaped, debossed “REGLAN” on one side and “ANI 10” on the opposite side
- In patients with a history of tardive dyskinesia (TD) or a dystonic reaction to metoclopramide
- When stimulation of gastrointestinal motility might be dangerous (e.g., in the presence of gastrointestinal hemorrhage, mechanical obstruction, or perforation).
- In patients with pheochromocytoma or other catecholamine-releasing paragangliomas. Reglan may cause a hypertensive/pheochromocytoma crisis, probably due to release of catecholamines from the tumor
- In patients with epilepsy. Reglan may increase the frequency and severity of seizures
- In patients with hypersensitivity to metoclopramide. Reactions have included laryngeal and glossal angioedema and bronchospasm
- Tardive dyskinesia
- Other extrapyramidal effects
- Neuroleptic malignant syndrome
- Depression
- Hypertension
- Fluid retention
- Hyperprolactinemia
- Effects on the ability to drive and operate machinery
- Tardive dyskinesia, acute dystonic reactions, drug-induced parkinsonism, akathisia, and other extrapyramidal symptoms
- Convulsive seizures
- Hallucinations
- Restlessness, drowsiness, fatigue, and lassitude occurred in approximately 10% of patients who received 10 mg four times daily. Insomnia, headache, confusion, dizziness, or depression with suicidal ideation occurred less frequently.
- Neuroleptic malignant syndrome, serotonin syndrome (in combination with serotonergic agents).
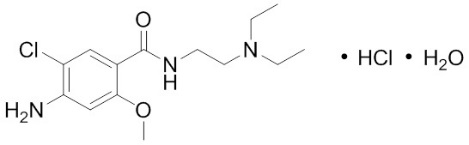
- Each Reglan 5 mg tablet contains 5 mg metoclopramide (equivalent to 5.91 mg of metoclopramide hydrochloride USP). Inactive ingredients consist of corn starch, D&C Yellow 10 Aluminum Lake, FD&C Blue 1 Aluminum Lake, lactose, microcrystalline cellulose, silicon dioxide, and stearic acid.
- Each Reglan 10 mg tablet contains 10 mg metoclopramide (equivalent to 11.82 mg metoclopramide hydrochloride USP). Inactive ingredients consist of magnesium stearate, mannitol, microcrystalline cellulose, and stearic acid.
- Tardive dyskinesia and other extrapyramidal reactions
- Neuroleptic malignant syndrome
- Depression and/or possible suicidal ideation