Brand Name
Spevigo
Generic Name
Spesolimab-Sbzo
View Brand Information FDA approval date: September 01, 2022
Classification: Interleukin-36 Receptor Antagonist
Form: Injection
What is Spevigo (Spesolimab-Sbzo)?
SPEVIGO is indicated for the treatment of generalized pustular psoriasis in adults and pediatric patients 12 years of age and older and weighing at least 40 kg. SPEVIGO is an interleukin-36 receptor antagonist indicated for the treatment of generalized pustular psoriasis in adults and pediatric patients 12 years of age and older and weighing at least 40 kg.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
SPEVIGO (spesolimab-sbzo)
1INDICATIONS AND USAGE
SPEVIGO is indicated for the treatment of generalized pustular psoriasis (GPP) in adults and pediatric patients 12 years of age and older and weighing at least 40 kg.
2DOSAGE FORMS AND STRENGTHS
SPEVIGO is a colorless to slightly brownish-yellow, clear to slightly opalescent solution available as:
3CONTRAINDICATIONS
SPEVIGO is contraindicated in patients with severe or life-threatening hypersensitivity to spesolimab-sbzo or to any of the excipients in SPEVIGO. Reported hypersensitivity reactions have included drug reaction with eosinophilia and systemic symptoms (DRESS) and anaphylaxis
4ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Infections
- Hypersensitivity and Infusion-Related Reactions
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
4.2Postmarketing Experience
The following adverse reactions have been identified during postapproval use of SPEVIGO. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune System Disorders: Immediate systemic hypersensitivity reactions, including anaphylaxis.
5DESCRIPTION
Spesolimab-sbzo, an interleukin-36 receptor antagonist, is a humanized monoclonal IgG1 antibody (mAb) against human IL-36R produced in Chinese hamster ovary (CHO) cells by recombinant DNA technology. Spesolimab-sbzo has a molecular weight of approximately 146 kDa.
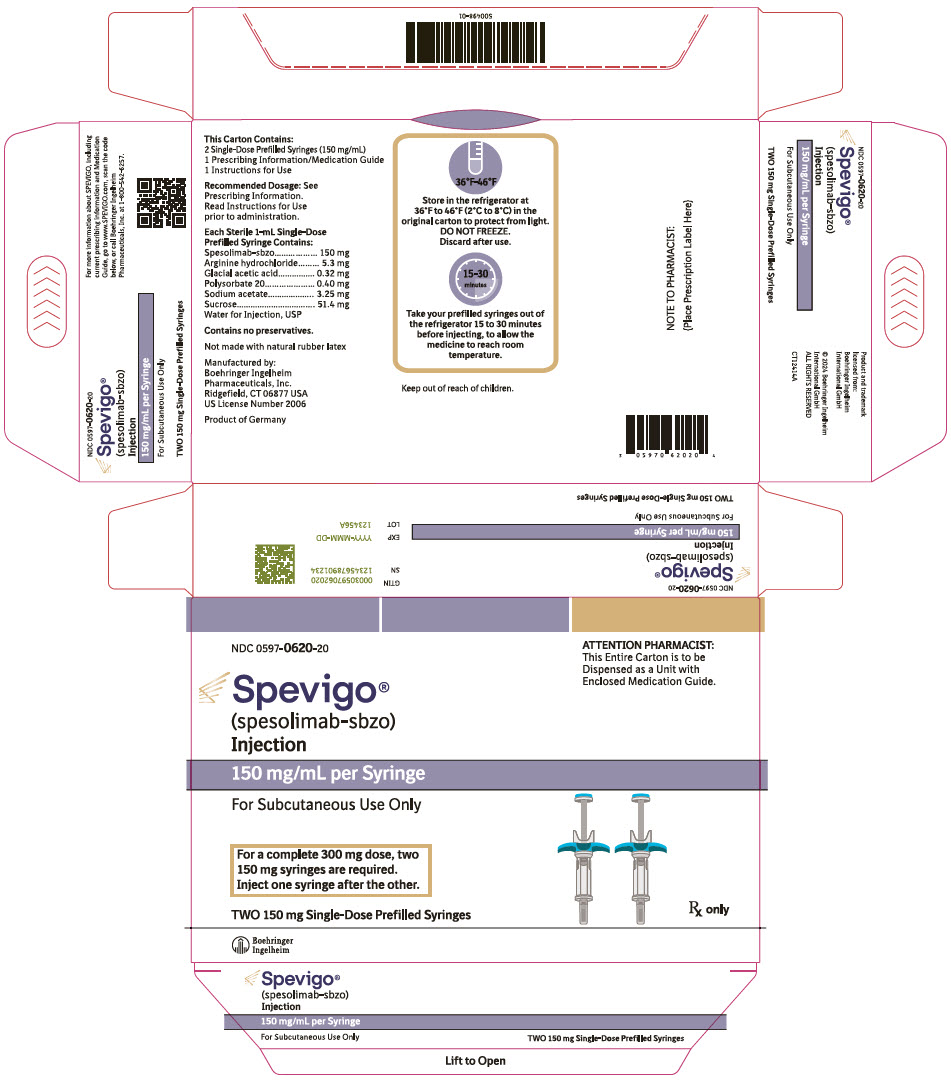
SPEVIGO (spesolimab-sbzo) injection is a sterile, preservative-free, colorless to slightly brownish-yellow, clear to slightly opalescent solution supplied in a single-dose prefilled syringe for subcutaneous use. Each 2 mL prefilled syringe contains 300 mg spesolimab-sbzo, arginine hydrochloride (10.6 mg), glacial acetic acid (0.64 mg), polysorbate 20 (0.80 mg), sodium acetate (6.50 mg), sucrose (102.8 mg), and Water for Injection, USP with a pH of 5.2 to 5.8. Each 1 mL prefilled syringe contains 150 mg spesolimab-sbzo, arginine hydrochloride (5.3 mg), glacial acetic acid (0.32 mg), polysorbate 20 (0.40 mg), sodium acetate (3.25 mg), sucrose (51.4 mg), and Water for Injection, USP with a pH of 5.2 to 5.8.
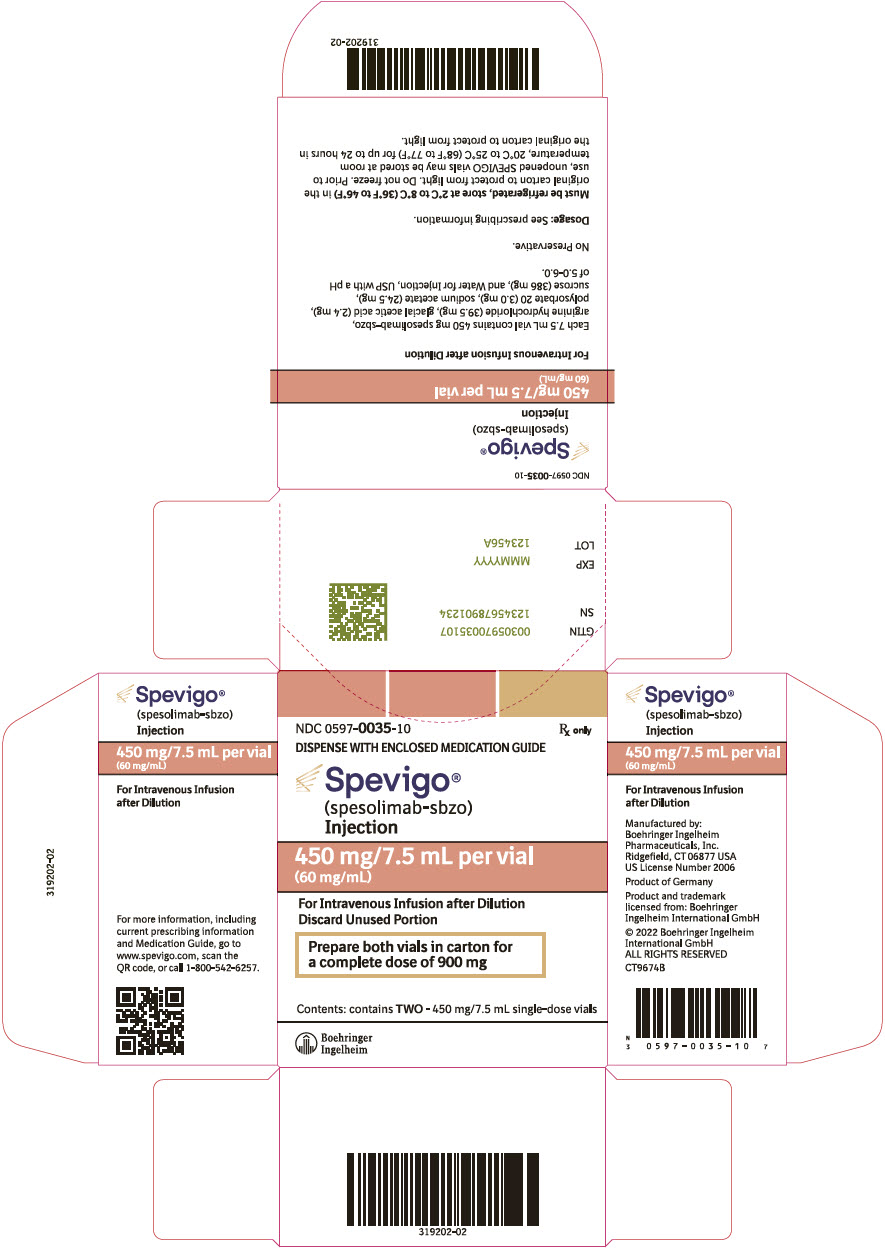
SPEVIGO (spesolimab-sbzo) injection is a sterile, preservative-free, colorless to slightly brownish-yellow, clear to slightly opalescent solution supplied in a single-dose vial for intravenous use. Each 7.5 mL vial contains 450 mg spesolimab-sbzo, arginine hydrochloride (39.5 mg), glacial acetic acid (2.4 mg), polysorbate 20 (3.0 mg), sodium acetate (24.5 mg), sucrose (386 mg), and Water for Injection, USP with a pH of 5.2 to 5.8.
6PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
7INSTRUCTIONS FOR USE SPEVIGO®(spea VEE go) (spesolimab-sbzo) injection, for subcutaneous use mg/mL Two 150 mg/mL single-dose prefilled syringes for a total of 300 mg dose
This Instructions for Use contains information on how to inject SPEVIGO.
Read
SPEVIGO is for one-time use only.
Your healthcare provider has prescribed a dose of SPEVIGO for you or your child that requires 2 injections (2 prefilled syringes) to deliver a complete dose.
You must inject the contents of both SPEVIGO prefilled syringes that come in the carton to deliver the complete dose.
Getting to know SPEVIGO:
SPEVIGO comes in a prefilled syringe with a safety cover. The needle is pulled back into the safety cover after injection.
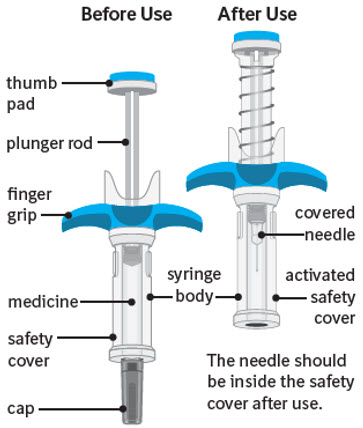
Guide to parts:
The figure below shows SPEVIGO before use, and after use with the activated safety cover.
Important information you need to know before injecting SPEVIGO:
- You must inject the contents of both SPEVIGO prefilled syringes to deliver a complete dose.
- Inspect the SPEVIGO carton to be sure that you have the correct medicine, 2 prefilled syringes for your or your child's prescribed dose, for any damage, and the expiration date (EXP).
- Do not use SPEVIGO if the liquid is cloudy or contains flakes or large or colored particles.
- Do not use SPEVIGO if the expiration date (EXP) has passed.
- Do not use SPEVIGO if the prefilled syringe has been dropped.
- Do not remove the cap until you are ready to inject.
- Inject SPEVIGO under the skin (subcutaneous injection) in either the upper thighs or stomach-area (abdomen). Do not inject SPEVIGO into any other area of the body.
Storing SPEVIGO:
Keep SPEVIGO and all medicines out of the reach of children.
Manufactured by:
Licensed from: Boehringer Ingelheim International GmbH, Ingelheim, Germany
Copyright © 2025 Boehringer Ingelheim International GmbH ALL RIGHTS RESERVED
For more information about SPEVIGO, including current prescribing information and Medication Guide, go to

This Instructions for Use has been approved by the U.S. Food and Drug Administration.
8INSTRUCTIONS FOR USE SPEVIGO®(spea VEE go) (spesolimab-sbzo) injection, for subcutaneous use mg/2 mL One 300 mg/2 mL single-dose prefilled syringe for a total of 300 mg dose
This Instructions for Use contains information on how to inject SPEVIGO.
Read
SPEVIGO is for one-time use only.
Your healthcare provider has prescribed a dose of SPEVIGO for you or your child that requires 1 injection to deliver a complete dose.
You must inject the contents of the SPEVIGO prefilled syringe that comes in the carton to deliver the complete dose.
Getting to know SPEVIGO:
SPEVIGO comes in a prefilled syringe with a safety cover. The needle is pulled back into the safety cover after injection.
Guide to parts:
The figure below shows SPEVIGO before use, and after use with the activated safety cover.
Important information you need to know before injecting SPEVIGO
- Inspect the SPEVIGO carton to be sure that you have the correct medicine, 1 prefilled syringe for your or your child's prescribed dose, for any damage, and the expiration date (EXP).
- Do not use SPEVIGO if the liquid is cloudy or contains flakes or large or colored particles.
- Do not use SPEVIGO if the expiration date (EXP) has passed.
- Do not use SPEVIGO if the prefilled syringe has been dropped.
- Do not remove the cap until you are ready to inject.
- Inject SPEVIGO under the skin (subcutaneous injection) in either the upper thighs or stomach-area (abdomen).
Storing SPEVIGO:
Keep SPEVIGO and all medicines out of the reach of children.
Manufactured by:
Licensed from: Boehringer Ingelheim International GmbH, Ingelheim, Germany
Copyright © 2025 Boehringer Ingelheim International GmbH ALL RIGHTS RESERVED
For more information about SPEVIGO, including current prescribing information and Medication Guide, go to

This Instructions for Use has been approved by the U.S. Food and Drug Administration.
9INSTRUCTIONS FOR USE SPEVIGO®(spea VEE go) (spesolimab-sbzo) injection, for subcutaneous use mg/2 mL Two 300 mg/2 mL single-dose prefilled syringes for a total of 600 mg dose
This Instructions for Use contains information on how to inject SPEVIGO.
Read
SPEVIGO is for one-time use only.
Your healthcare provider has prescribed a dose of SPEVIGO for you or your child that requires 2 injections (2 prefilled syringes) to deliver a complete dose.
You must inject the contents of both SPEVIGO prefilled syringes that come in the carton to deliver the complete dose.

Getting to know SPEVIGO:
SPEVIGO comes in a prefilled syringe with a safety cover. The needle is pulled back into the safety cover after injection.
Guide to parts:
The figure below shows SPEVIGO before use, and after use with the activated safety cover.

Important information you need to know before injecting SPEVIGO
- You must inject the contents of both SPEVIGO prefilled syringes to deliver a complete dose.
- Inspect the SPEVIGO carton to be sure that you have the correct medicine, 2 prefilled syringes for your or your child's prescribed dose, for any damage, and the expiration date (EXP).
- Do not use SPEVIGO if the liquid is cloudy or contains flakes or large or colored particles.
- Do not use SPEVIGO if the expiration date (EXP) has passed.
- Do not use SPEVIGO if the prefilled syringe has been dropped.
- Do not remove the cap until you are ready to inject.
- Inject SPEVIGO under the skin (subcutaneous injection) in either the upper thighs or stomach-area (abdomen). Do not inject SPEVIGO into any other area of the body.
Storing SPEVIGO
Keep SPEVIGO and all medicines out of the reach of children.
Manufactured by:
Licensed from: Boehringer Ingelheim International GmbH, Ingelheim, Germany
Copyright © 2025 Boehringer Ingelheim International GmbH ALL RIGHTS RESERVED
For more information about SPEVIGO, including current prescribing information and Medication Guide, go to

This Instructions for Use has been approved by the U.S. Food and Drug Administration.
10PRINCIPAL DISPLAY PANEL - 450 mg/7.5 mL Vial Carton
NDC 0597-0035-10
DISPENSE WITH ENCLOSED MEDICATION GUIDE
Spevigo
450 mg/7.5 mL per vial
For Intravenous Infusion after Dilution
Prepare both vials in carton for
Contents: contains TWO - 450 mg/7.5 mL single-dose vials
Boehringer

11PRINCIPAL DISPLAY PANEL - 150 mg Syringe Carton
NDC 0597-0620-20
ATTENTION PHARMACIST:
Spevigo
150 mg/mL per Syringe
For Subcutaneous Use Only
For a complete 300 mg dose, two
TWO 150 mg Single-Dose Prefilled Syringes
Rx only
Boehringer

12PRINCIPAL DISPLAY PANEL - 300 mg/2 mL Syringe Carton
NDC 0597-7705-41
ATTENTION PHARMACIST:
Spevigo
300 mg/2 mL
For Subcutaneous Use Only
1 Single-Dose Prefilled Syringe
Rx only
Boehringer
