Generic Name
Ruxolitinib
Brand Names
Jakafi, Opzelura
FDA approval date: November 16, 2011
Classification: Janus Kinase Inhibitor
Form: Cream, Tablet
What is Jakafi (Ruxolitinib)?
OPZELURA is a Janus kinase inhibitor indicated for: the topical short-term and non-continuous chronic treatment of mild to moderate atopic dermatitis in non-immunocompromised adult and pediatric patients 2 years of age and older whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
JAKAFI (ruxolitinib)
1DOSAGE FORMS AND STRENGTHS
5 mg tablets - round and white with "INCY" on one side and "5" on the other.
10 mg tablets - round and white with "INCY" on one side and "10" on the other.
15 mg tablets - oval and white with "INCY" on one side and "15" on the other.
20 mg tablets - capsule-shaped and white with "INCY" on one side and "20" on the other.
25 mg tablets - oval and white with "INCY" on one side and "25" on the other.
2CONTRAINDICATIONS
None.
3ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling:
- Thrombocytopenia, Anemia and Neutropenia
- Risk of Infection
- Symptom Exacerbation Following Interruption or Discontinuation of Treatment with Jakafi
- Non-Melanoma Skin Cancer
- Lipid Elevations
- Major Adverse Cardiovascular Events (MACE)
- Thrombosis
- Secondary Malignancies
3.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Myelofibrosis
The safety of Jakafi was assessed in 617 patients in six clinical studies with a median duration of follow-up of 10.9 months, including 301 patients with MF in two Phase 3 studies.
In these two Phase 3 studies, patients had a median duration of exposure to Jakafi of 9.5 months (range 0.5 to 17 months), with 89% of patients treated for more than 6 months and 25% treated for more than 12 months. One hundred and eleven (111) patients started treatment at 15 mg twice daily and 190 patients started at 20 mg twice daily. In patients starting treatment with 15 mg twice daily (pretreatment platelet counts of 100 to 200 x 10
In a double-blind, randomized, placebo-controlled study of Jakafi, among the 155 patients treated with Jakafi, the most frequent adverse reactions were thrombocytopenia and anemia
Discontinuation for adverse events, regardless of causality, was observed in 11% of patients treated with Jakafi and 11% of patients treated with placebo.
Table 12 presents the most common nonhematologic adverse reactions occurring in patients who received Jakafi in the double-blind, placebo-controlled study during randomized treatment.
Description of Selected Adverse Reactions
Anemia
In the two Phase 3 clinical studies, median time to onset of first CTCAE Grade 2 or higher anemia was approximately 6 weeks. One patient (< 1%) discontinued treatment because of anemia. In patients receiving Jakafi, mean decreases in hemoglobin reached a nadir of approximately 1.5 to 2.0 g/dL below baseline after 8 to 12 weeks of therapy and then gradually recovered to reach a new steady state that was approximately 1.0 g/dL below baseline. This pattern was observed in patients regardless of whether they had received transfusions during therapy.
In the randomized, placebo-controlled study, 60% of patients treated with Jakafi and 38% of patients receiving placebo received red blood cell transfusions during randomized treatment. Among transfused patients, the median number of units transfused per month was 1.2 in patients treated with Jakafi and 1.7 in placebo treated patients.
Thrombocytopenia
In the two Phase 3 clinical studies, in patients who developed Grade 3 or 4 thrombocytopenia, the median time to onset was approximately 8 weeks. Thrombocytopenia was generally reversible with dose reduction or dose interruption. The median time to recovery of platelet counts above 50 x 10
Neutropenia
In the two Phase 3 clinical studies, 1% of patients reduced or stopped Jakafi because of neutropenia.
Table 13 provides the frequency and severity of clinical hematology abnormalities reported for patients receiving treatment with Jakafi or placebo in the placebo-controlled study.
Additional Data from the Placebo-Controlled Study
- 25% of patients treated with Jakafi and 7% of patients treated with placebo developed newly occurring or worsening Grade 1 abnormalities in alanine transaminase (ALT). The incidence of greater than or equal to Grade 2 elevations was 2% for Jakafi with 1% Grade 3 and no Grade 4 ALT elevations.
- 17% of patients treated with Jakafi and 6% of patients treated with placebo developed newly occurring or worsening Grade 1 abnormalities in aspartate transaminase (AST). The incidence of Grade 2 AST elevations was < 1% for Jakafi with no Grade 3 or 4 AST elevations.
- 17% of patients treated with Jakafi and < 1% of patients treated with placebo developed newly occurring or worsening Grade 1 elevations in cholesterol. The incidence of Grade 2 cholesterol elevations was < 1% for Jakafi with no Grade 3 or 4 cholesterol elevations.
Polycythemia Vera
In a randomized, open-label, active-controlled study, 110 patients with PV resistant to or intolerant of hydroxyurea received Jakafi and 111 patients received best available therapy
Clinically relevant laboratory abnormalities are shown in Table 15.
Acute Graft-Versus-Host Disease
In a single-arm, open-label study, 71 adults (ages 18-73 years) were treated with Jakafi for aGVHD failing treatment with steroids with or without other immunosuppressive drugs
There were no fatal adverse reactions to Jakafi. An adverse reaction resulting in treatment discontinuation occurred in 31% of patients. The most common adverse reaction leading to treatment discontinuation was infection (10%). Table 16 shows the adverse reactions other than laboratory abnormalities.
Selected laboratory abnormalities during treatment with Jakafi are shown in Table 17.
Chronic Graft-Versus-Host Disease
In a Phase 3, randomized, open-label, multi-center study, 165 patients were treated with Jakafi and 158 patients were treated with best available therapy for cGVHD failing treatment with steroids with or without other immunosuppressive drugs
There were five fatal adverse reactions to Jakafi, including 1 from toxic epidermal necrolysis and 4 from neutropenia, anemia and/or thrombocytopenia. An adverse reaction resulting in treatment discontinuation occurred in 18% of patients treated with Jakafi. An adverse reaction resulting in dose modification occurred in 27%, and an adverse reaction resulting in treatment interruption occurred in 23%. The most common hematologic adverse reactions (incidence > 35%) are anemia and thrombocytopenia. The most common nonhematologic adverse reactions (incidence ≥ 20%) are infections (pathogen not specified) and viral infection.
Table 18 presents the most frequent nonlaboratory adverse reactions occurring up to Cycle 7 Day 1 of randomized treatment.
Clinically relevant laboratory abnormalities are shown in Table 19.
3.2Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Jakafi. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
- Infections and Infestations: Herpes simplex virus reactivation and/or dissemination
4OVERDOSAGE
There is no known antidote for overdoses with Jakafi. Single doses up to 200 mg have been given with acceptable acute tolerability. Higher than recommended repeat doses are associated with increased myelosuppression including leukopenia, anemia and thrombocytopenia. Appropriate supportive treatment should be given.
Hemodialysis is not expected to enhance the elimination of Jakafi.
5DESCRIPTION
Ruxolitinib phosphate is a kinase inhibitor with the chemical name (
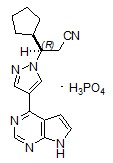
Ruxolitinib phosphate is a white to off-white to light pink powder and is soluble in aqueous buffers across a pH range of 1 to 8.
Jakafi (ruxolitinib) Tablets are for oral administration. Each tablet contains 6.6 mg, 13.2 mg, 19.8 mg, 26.4 mg, or 33 mg of ruxolitinib phosphate equivalent to 5 mg, 10 mg, 15 mg, 20 mg, or 25 mg of ruxolitinib free base, respectively, together with microcrystalline cellulose, lactose monohydrate, magnesium stearate, colloidal silicon dioxide, sodium starch glycolate, povidone and hydroxypropyl cellulose.
6HOW SUPPLIED/STORAGE AND HANDLING
Jakafi (ruxolitinib) Tablets are available as follows:
7PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Thrombocytopenia, Anemia and Neutropenia
Inform patients that Jakafi is associated with thrombocytopenia, anemia and neutropenia, and of the need to monitor complete blood counts before and during treatment. Advise patients to observe for and report bleeding
Infections
Inform patients of the signs and symptoms of infection and to report any such signs and symptoms promptly.
Inform patients regarding the early signs and symptoms of herpes zoster and of progressive multifocal leukoencephalopathy, and advise patients to seek advice of a clinician if such symptoms are observed
Symptom Exacerbation Following Interruption or Discontinuation of Treatment with Jakafi
Inform patients that after discontinuation of treatment, signs and symptoms from myeloproliferative neoplasms are expected to return. Instruct patients not to interrupt or discontinue Jakafi therapy without consulting their physician
Non-Melanoma Skin Cancer
Inform patients that Jakafi may increase their risk of certain non-melanoma skin cancers. Advise patients to inform their healthcare provider if they have ever had any type of skin cancer or if they observe any new or changing skin lesions
Lipid Elevations
Inform patients that Jakafi may increase blood cholesterol, and of the need to monitor blood cholesterol levels
Major Adverse Cardiovascular Events (MACE)
Advise patients that events of major adverse cardiovascular events (MACE) including myocardial infarction, stroke, and cardiovascular death, have been reported in clinical studies with another JAK-inhibitor used to treat rheumatoid arthritis, a condition for which Jakafi is not indicated. Advise patients, especially current or past smokers or patients with other cardiovascular risk factors, to be alert for the development of signs and symptoms of cardiovascular events
Thrombosis
Advise patients that events of DVT and PE have been reported in clinical studies with another JAK-inhibitor used to treat rheumatoid arthritis, a condition for which Jakafi is not indicated. Advise patients to tell their healthcare provider if they develop any signs or symptoms of a DVT or PE
Secondary Malignancies
Advise patients, especially current or past smokers and patients with a known secondary malignancy (other than a successfully treated NMSC), that lymphoma and other malignancies (excluding NMSC) have been reported in clinical studies with another JAK-inhibitor used to treat rheumatoid arthritis, a condition for which Jakafi is not indicated
Drug-Drug Interactions
Advise patients to inform their healthcare providers of all medications they are taking, including over-the-counter medications, herbal products and dietary supplements
Dialysis
Inform patients on dialysis that their dose should not be taken before dialysis but only following dialysis
Lactation
Inform women not to breastfeed during treatment with Jakafi and for two weeks after the final dose
Compliance
Advise patients to continue taking Jakafi every day for as long as their physician tells them and that this is a long-term treatment. Patients should not change dose or stop taking Jakafi without first consulting their physician. Patients should be aware that after discontinuation of treatment, signs and symptoms from myeloproliferative neoplasms are expected to return.
Manufactured for:
Jakafi is a registered trademark of Incyte. All rights reserved.
8mg Tablet Bottle Label
Rx only
NDC 50881-005-60
Jakafi® (Ruxolitinib) Tablets
5 mg
60 tablets
Each tablet contains 6.6 mg ruxolitinib phosphate equivalent to 5 mg ruxolitinib free base.

9mg Tablet Bottle Label
Rx only
NDC 50881-010-60
Jakafi® (Ruxolitinib) Tablets
10 mg
60 tablets
Each tablet contains 13.2 mg ruxolitinib phosphate equivalent to 10 mg ruxolitinib free base.

10mg Tablet Bottle Label
Rx only
NDC 50881-015-60
Jakafi® (Ruxolitinib) Tablets
15 mg
60 tablets
Each tablet contains 19.8 mg ruxolitinib phosphate equivalent to 15 mg ruxolitinib free base.

11mg Tablet Bottle Label
Rx only
NDC 50881-020-60
Jakafi® (Ruxolitinib) Tablets
20 mg
60 tablets
Each tablet contains 26.4 mg ruxolitinib phosphate equivalent to 20 mg ruxolitinib free base.

12mg Tablet Bottle Label
Rx only
NDC 50881-025-60
Jakafi® (Ruxolitinib) Tablets
25 mg
60 tablets
Each tablet contains 33 mg ruxolitinib phosphate equivalent to 25 mg ruxolitinib free base.
