Generic Name
Metoprolol
Brand Names
Lopressor, Toprol, Kapspargo
FDA approval date: December 23, 1993
Classification: beta-Adrenergic Blocker
Form: Injection, Tablet, Capsule, Solution
What is Lopressor (Metoprolol)?
Hypertension Metoprolol tartrate tablets are indicated for the treatment of hypertension. They may be used alone or in combination with other antihypertensive agents. Angina Pectoris Metoprolol tartrate tablets are indicated in the long-term treatment of angina pectoris. Myocardial Infarction Metoprolol tartrate tablets are indicated in the treatment of hemodynamically stable patients with definite or suspected acute myocardial infarction to reduce cardiovascular mortality when used alone or in conjunction with intravenous metoprolol tartrate. Oral metoprolol tartrate therapy can be initiated after intravenous metoprolol tartrate therapy, or alternatively, oral treatment can begin within 3 to 10 days of acute event.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Lopressor (metoprolol tartrate)
1DOSAGE FORMS AND STRENGTHS
LOPRESSOR is supplied as:
50 mg tablet – capsule shaped, biconvex, pink, scored (imprinted GEIGY on one side and 51 twice on the scored side)
100 mg tablet – capsule shaped, biconvex, light blue, scored (imprinted with GEIGY on one side and 71 twice on the scored side)
2CONTRAINDICATIONS
LOPRESSOR is contraindicated in severe bradycardia, second- or third-degree heart block, cardiogenic shock, systolic blood pressure <100, decompensated heart failure, sick sinus syndrome (unless a permanent pacemaker is in place), and in patients who are hypersensitive to any component of this product.
3ADVERSE REACTIONS
The following adverse reactions are described elsewhere in labeling:
- Worsening angina or myocardial infarction
- Worsening heart failure
- Worsening AV block
3.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Hypertension and Angina
Most adverse effects have been mild and transient.
Central Nervous System: Tiredness and dizziness have occurred in about 10% of patients. Depression has been reported in about 5 of 100 patients. Mental confusion and short-term memory loss have been reported. Headache, nightmares, and insomnia have also been reported.
Cardiovascular: Shortness of breath and bradycardia have occurred in approximately 3% of patients. Cold extremities; arterial insufficiency, usually of the Raynaud type; palpitations; heart failure exacerbations; peripheral edema; and hypotension have been reported in about 1% of patients. Gangrene in patients with pre-existing severe peripheral circulatory disorders has also been reported. [see Contraindications (4) and Warnings and Precautions (5.2)].
Respiratory: Wheezing (bronchospasm) and dyspnea have been reported in about 1% of patients [see Warnings and Precautions (5.3)]. Rhinitis has also been reported.
Gastrointestinal: Diarrhea has occurred in about 5% of patients. Nausea, dry mouth, gastric pain, constipation, flatulence, and heartburn have been reported in about 1% of patients. Vomiting was a common occurrence.
HypersensitiveReactions: Pruritus or rash have occurred in about 5% of patients. Photosensitivity and worsening of psoriasis has been reported.
Miscellaneous: Peyronie’s disease, musculoskeletal pain, blurred vision, and tinnitus has been reported.
Myocardial Infarction
In general, the adverse reactions observed in trials with metoprolol in MI are consistent with the hypertension and angina experience.
In a randomized comparison of Lopressor and placebo in the setting of acute MI the following adverse reactions were reported:
3.2Post-Marketing Experience
The following adverse reactions have been identified during post approval use of LOPRESSOR. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Central Nervous System: Reversible mental depression progressing to catatonia; an acute reversible syndrome characterized by disorientation for time and place, short-term memory loss, emotional lability, slightly clouded sensorium, and decreased performance on neuropsychometrics.
Hematologic: Agranulocytosis, nonthrombocytopenic purpura and thrombocytopenic purpura.
Hypersensitive Reactions: Fever combined with aching and sore throat, laryngospasm and respiratory distress.
Laboratory Findings:
Increase in blood triglycerides, elevated transaminase and decrease in High Density Lipoprotein (HDL)
4OVERDOSAGE
Signs and Symptoms - Overdosage of LOPRESSOR may lead to severe bradycardia, hypotension, and cardiogenic shock. Clinical presentation can also include: atrioventricular block, heart failure, bronchospasm, hypoxia, impairment of consciousness/coma, nausea and vomiting.
Treatment – Consider treating the patient with intensive care. Patients with myocardial infarction or heart failure may be prone to significant hemodynamic instability. Beta-blocker overdose may result in significant resistance to resuscitation with adrenergic agents, including beta-agonists. On the basis of the pharmacologic actions of metoprolol, employ the following measures.
Hemodialysis is unlikely to make a useful contribution to metoprolol elimination
Bradycardia: Evaluate the need for atropine, adrenergic-stimulating drugs or pacemaker to treat bradycardia and conduction disorders.
Hypotension: Treat underlying bradycardia. Consider intravenous vasopressor infusion, such as dopamine or norepinephrine.
Heart failure and shock: May be treated when appropriate with suitable volume expansion, injection of glucagon (if necessary, followed by an intravenous infusion of glucagon), intravenous administration of adrenergic drugs such as dobutamine, with α
Bronchospasm: Can usually be reversed by bronchodilators.
5DESCRIPTION
Lopressor tablets contain metoprolol tartrate, a selective beta
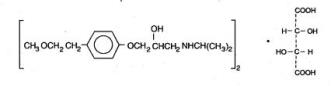
Metoprolol tartrate USP is a white, practically odorless, crystalline powder with a molecular weight of 684.82. It is very soluble in water; freely soluble in methylene chloride, in chloroform, and in alcohol; slightly soluble in acetone; and insoluble in ether.
Lopressor is available as 50 mg and 100 mg tablets for oral administration containing 50 mg and 100 mg metoprolol tartrate, respectively.
InactiveIngredients: Tablets contain microcrystalline cellulose, colloidal silicon dioxide, lactose monohydrate, magnesium stearate, povidone, sodium starch glycolate. Film coating contains Opadry YS-1-1419 Pink (50 mg tablets) or Opadry YS-1-4281 Blue (100 mg tablets).
6HOW SUPPLIED/STORAGE AND HANDLING
Lopressor (metoprolol tartrate)tablets
Tablets 50 mg – capsule-shaped, biconvex, pink, scored (imprinted LOPRESSOR on one side and 458 twice on the scored side)
Bottles of 100………………………………………….………NDC 30698-458-01
Tablets 100 mg – capsule-shaped, biconvex, light blue, scored (imprinted LOPRESSOR on one side and 459 twice on the scored side)
Bottles of 100…………………………………………………. NDC 30698-459-01
Storage:
Store at 77°F (25°C); excursions permitted to 59° to 86°F (15° to 30°C) [See USP Controlled Room Temperature]. Protect from moisture and heat.
Store at 77°F (25°C); excursions permitted to 59° to 86°F (15° to 30°C) [See USP Controlled Room Temperature]. Protect from moisture and heat.
Dispense in a tight, light-resistant container (USP).
7PATIENT COUNSELING INFORMATION
Advise patients to take LOPRESSOR regularly and continuously, as directed, preferably with or immediately following meals. If a dose is missed, the patient should take only the next scheduled dose (without doubling it). Patients should not interrupt or discontinue LOPRESSOR without consulting the physician.
Advise patients (1) to avoid operating automobiles and machinery or engaging in other tasks requiring alertness until the patient’s response to therapy with LOPRESSOR has been determined; (2) to contact the physician if any difficulty in breathing occurs; (3) to inform the physician or dentist before any type of surgery that he or she is taking LOPRESSOR.
Inform patients or caregivers that there is a risk of hypoglycemia when LOPRESSOR is given to patients who are fasting or who are vomiting. Monitor for symptoms of hypoglycemia.
Manufactured for and Distributed by:
Validus Pharmaceuticals LLC
Parsippany, NJ 07054
info@validuspharma.com
www.validuspharma.com
1-866-982-5438
Validus Pharmaceuticals LLC
Parsippany, NJ 07054
info@validuspharma.com
www.validuspharma.com
1-866-982-5438
Product of Spain
© 2023 Validus Pharmaceuticals LLC
60025-06