Generic Name
MetFORMIN
Brand Names
Alogliptin, Glyburide, Jentadueto, Glipizide, Janumet, Pioglitazole, Glumetza, Pioglitazone, Riomet, Sitagliptin, Saxagliptin, Glyburide-MetFORMIN, Zituvimet, Kazano, Synjardy, Dapagliflozin, Actoplus, Invokamet, Xigduo, Trijardy, Segluromet, MetFORMIN Hydrochoride, Kombiglyze
FDA approval date: January 24, 2002
Classification: Dipeptidyl Peptidase 4 Inhibitor
Form: Tablet, Solution
What is Alogliptin (MetFORMIN)?
Metformin hydrochloride extended-release tablets, USP are indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. Metformin hydrochloride is a biguanide indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
alogliptin and metformin hydrochloride (alogliptin and metformin hydrochloride)
WARNING: LACTIC ACIDOSIS
Postmarketing cases of metformin-associated lactic acidosis have resulted in death, hypothermia, hypotension, and resistant bradyarrhythmias. The onset of metformin-associated lactic acidosis is often subtle, accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, somnolence, and abdominal pain. Metformin-associated lactic acidosis was characterized by elevated blood lactate levels (greater than 5 mmol/L), anion gap acidosis (without evidence of ketonuria or ketonemia), an increased lactate/pyruvate ratio; and metformin plasma levels generally greater than 5 mcg/mL
Risk factors for metformin-associated lactic acidosis include renal impairment, concomitant use of certain drugs (e.g., carbonic anhydrase inhibitors such as topiramate), age 65 years old or greater, having a radiological study with contrast, surgery and other procedures, hypoxic states (e.g., acute congestive heart failure), excessive alcohol intake, and hepatic impairment.
Steps to reduce the risk of and manage metformin-associated lactic acidosis in these high risk groups are provided in the Full Prescribing Information
If metformin-associated lactic acidosis is suspected, immediately discontinue alogliptin and metformin HCl tablets and institute general supportive measures in a hospital setting. Prompt hemodialysis is recommended
1INDICATIONS AND USAGE
Alogliptin and metformin HCl tablets are indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
2DOSAGE FORMS AND STRENGTHS
- 12.5 mg/500 mg tablets are pale yellow, oblong, film-coated tablets with "12.5/500" debossed on one side and "322M" debossed on the other side
- 12.5 mg/1000 mg tablets are pale yellow, oblong, film-coated tablets with "12.5/1000" debossed on one side and "322M" debossed on the other side
3CONTRAINDICATIONS
Alogliptin and metformin HCl tablets are contraindicated in patients with:
- Severe renal impairment (eGFR below 30 mL/min/1.73 m
- Acute or chronic metabolic acidosis, including diabetic ketoacidosis with or without coma.
- History of serious hypersensitivity reaction to alogliptin or metformin or any of the excipients, such as anaphylaxis, angioedema and severe cutaneous adverse reactions
4ADVERSE REACTIONS
The following serious adverse reactions are described below or elsewhere in the prescribing information:
- Pancreatitis
- Heart Failure
- Hypersensitivity Reactions
- Hepatic Effects
- Severe and Disabling Arthralgia
- Bullous Pemphigoid
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
4.2Postmarketing Experience
The following adverse reactions have been identified during postmarketing use. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
5DESCRIPTION
Alogliptin and metformin HCl tablets contain two oral antihyperglycemic drugs used in the management of type 2 diabetes mellitus: alogliptin and metformin HCl.
6HOW SUPPLIED/STORAGE AND HANDLING
Alogliptin and metformin HCl tablets are available in the following strengths and packages: 12.5 mg/500 mg tablet: pale yellow, oblong, film-coated tablets with "12.5/500" debossed on one side and "322M" debossed on the other side, available in:
12.5 mg/1000 mg tablet: pale yellow, oblong, film-coated tablets with "12.5/1000" debossed on one side and "322M" debossed on the other side, available in:
7PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
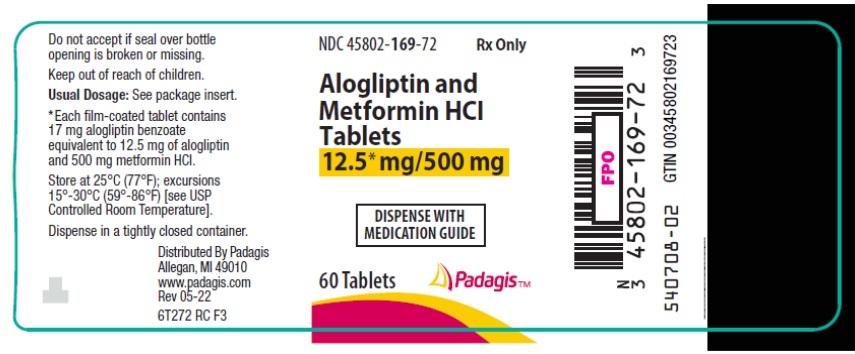
8PRINCIPAL DISPLAY PANEL - 12.5 mg/500 mg Tablet Bottle Label
NDC 45802-
Rx Only
Alogliptin and
DISPENSE WITH
60 Tablets
Padagis
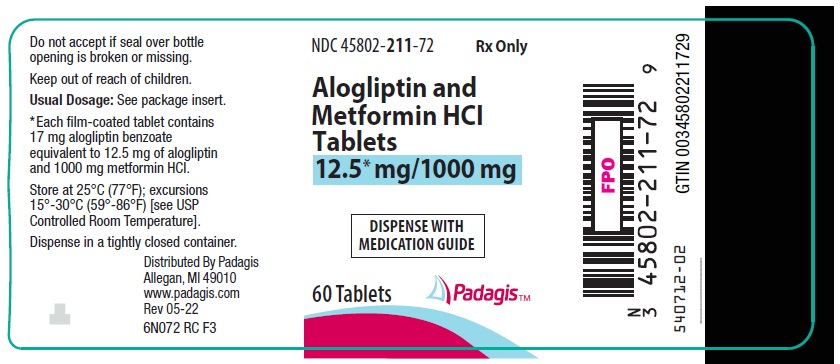
9PRINCIPAL DISPLAY PANEL - 12.5 mg/1000 mg Tablet Bottle Label
NDC 45802-
Rx Only
Alogliptin and
DISPENSE WITH
60 Tablets
Padagis