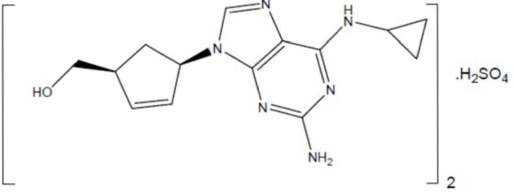
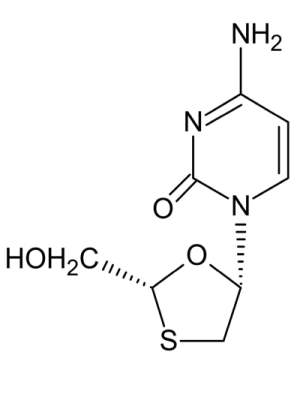
Lamivudine
What is Abacavir (Lamivudine)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: Phase III trial evaluating doravirine as an alternative to dolutegravir in treatment naïve people living with HIV-1 infection.
Summary: This phase II trial studies the effect of lamivudine in combination with standard of care chemoimmunotherapy in treating patients with extensive stage small cell lung cancer. Even though small cell lung cancer is initially highly responsive to first-line chemotherapy treatment, treatment resistance inevitably emerges; treatment resistance is when tumor cells stop responding to a drug treatment tha...
Summary: This is a Phase II, open-label, seven-arm parallel study evaluating the efficacy and safety of combined treatment (sodium cromoglicate, choline, efavirenz, SHR 1811, SHR 2102, mecapegfilgrastim, theophylline) with immune checkpoint inhibitor or immune checkpoint inhibitor rechallenge(AK131) in mTNBC (triple negative breast cancer) patients who progressed during previous immune checkpoint inhibitor...
Related Latest Advances
Brand Information
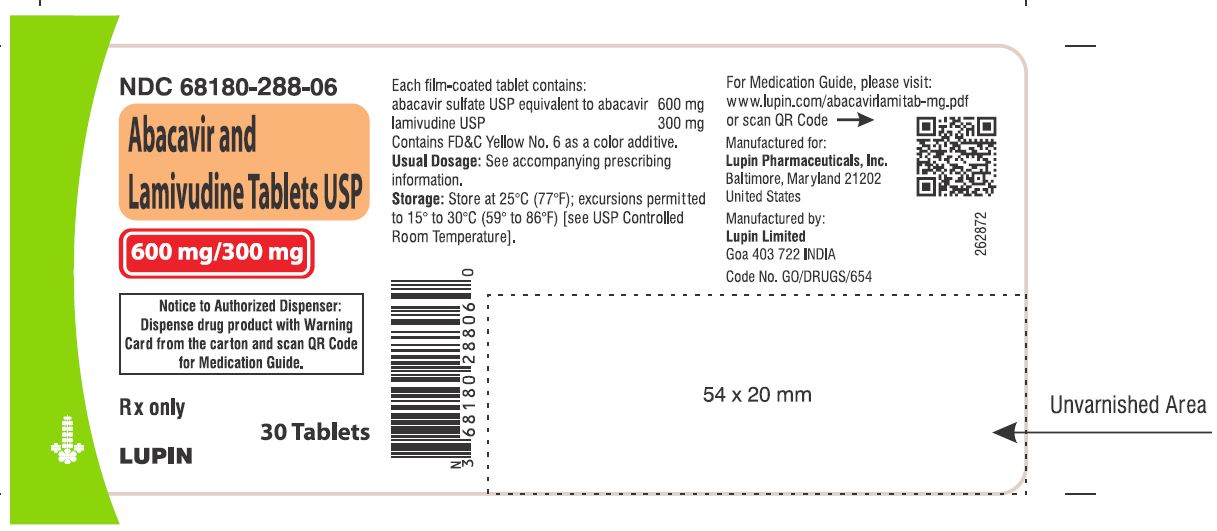
- that a Medication Guide and Warning Card summarizing the symptoms of the abacavir hypersensitivity reaction and other product information will be dispensed by the pharmacist with each new prescription and refill of abacavir and lamivudine tablets, and instruct the patient to read the Medication Guide and Warning Card every time to obtain any new information that may be present about abacavir and lamivudine tablets. The complete text of the Medication Guide is reprinted at the end of this document.
- to carry the Warning Card with them.
- how to identify a hypersensitivity reaction
- that if they develop symptoms consistent with a hypersensitivity reaction they should call their healthcare provider right away to determine if they should stop taking abacavir and lamivudine tablets.
- that a hypersensitivity reaction can worsen and lead to hospitalization or death if abacavir and lamivudine tablets is not immediately discontinued.
- to not restart abacavir and lamivudine tablets or any other abacavir-containing product following a hypersensitivity reaction because more severe symptoms can occur within hours and may include life-threatening hypotension and death.
- that if they have a hypersensitivity reaction, they should dispose of any unused abacavir and lamivudine tablets to avoid restarting abacavir.
- that a hypersensitivity reaction is usually reversible if it is detected promptly and abacavir and lamivudine tablets is stopped right away.
- that if they have interrupted abacavir and lamivudine tablets for reasons other than symptoms of hypersensitivity (for example, those who have an interruption in drug supply), a serious or fatal hypersensitivity reaction may occur with reintroduction of abacavir.
- to not restart abacavir and lamivudine tablets or any other abacavir-containing product without medical consultation and only if medical care can be readily accessed by the patient or others.