Altoprev
What is Altoprev (Lovastatin)?
Approved To Treat
Related Clinical Trials
Summary: This study will evaluate whether simvastatin reduces intraprostatic immunosuppressive microenvironment through YAP-mediated T-reg dysfunction, and increases intraprostatic anti-tumor immune response in men recently diagnosed with localized prostate cancer electing to receive prostatectomy for their care. Half the men will be randomized to receive statins for 8 weeks prior to their surgery, while t...
Summary: This study has the purpose to answer how the Lipoprotein(a) (Lp(a)) level is distributed among Atherosclerotic cardiovascular disease (ASCVD) patients in Russia, and what is the connection between elevated levels of this parameter and the cardiovascular disease (CVD) risk.
Summary: This is a proof-of-concept, Open label, randomized, multicentric, superiority phase-2 study.
Related Latest Advances
Brand Information
- To reduce the risk of myocardial infarction, unstable angina, and coronary revascularization procedures in adults at high risk for coronary heart disease.
- As an adjunct to diet to reduce low-density lipoprotein cholesterol (LDL-C) and slow the progression of coronary atherosclerosis in adults with coronary heart disease.
- As an adjunct to diet to reduce LDL-C in adults with primary hyperlipidemia, including heterozygous familial hypercholesterolemia (HeFH).
- Concomitant administration of strong CYP3A inhibitors and erythromycin
- Acute liver failure or decompensated cirrhosis
- Hypersensitivity to lovastatin or any excipients in Altoprev. Hypersensitivity reactions, including anaphylaxis, angioedema and Stevens-Johnson syndrome, have been reported
- Myopathy and Rhabdomyolysis
- Immune-Mediated Necrotizing Myopathy
- Hepatic Dysfunction
- Increases in HbA1c and Fasting Serum Glucose Levels
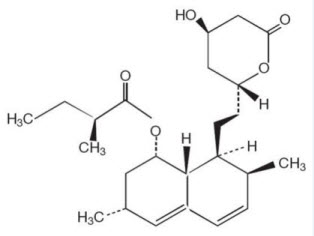
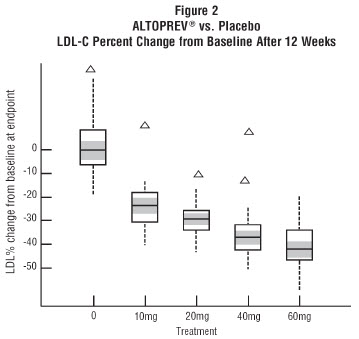
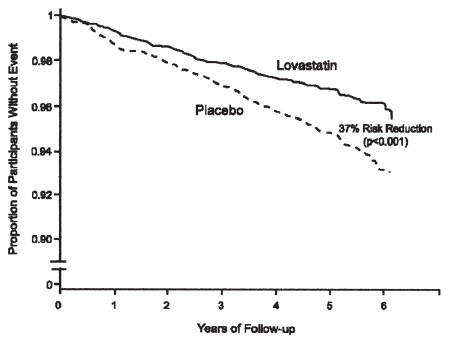

(lovastatin)
extended-release tablets

(lovastatin)
extended-release tablets

(lovastatin)
extended-release tablets



