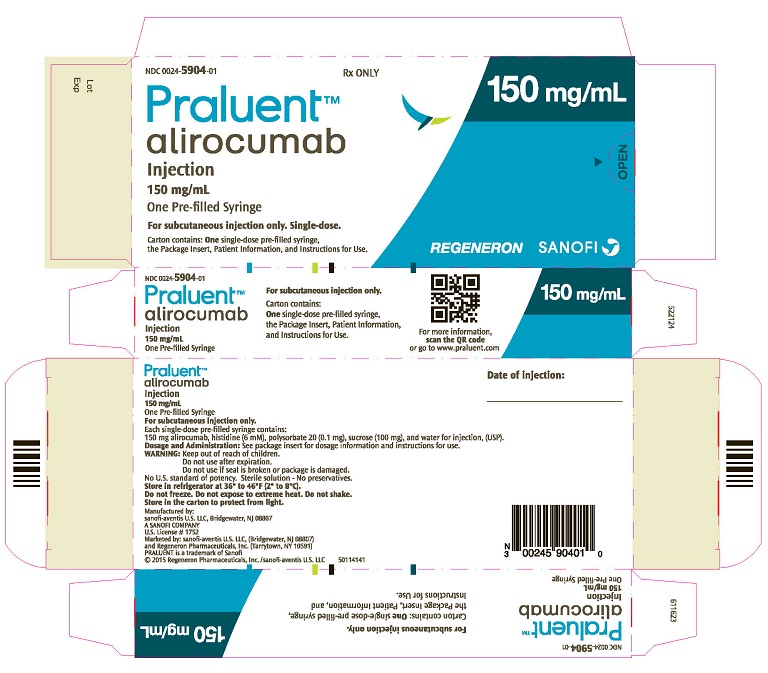
Praluent
What is Praluent (Alirocumab)?
Approved To Treat
Related Clinical Trials
Summary: This study has the purpose to answer how the Lipoprotein(a) (Lp(a)) level is distributed among Atherosclerotic cardiovascular disease (ASCVD) patients in Russia, and what is the connection between elevated levels of this parameter and the cardiovascular disease (CVD) risk.
Background: Drinking alcohol can lead to swelling and injury in the liver. Long-term heavy drinking may lead to liver disease. Researchers want to study the relationship between a drug called alirocumab, alcohol use, and liver functioning/swelling.
Summary: In this open-label, two-arm, randomized phase 2 clinical trial, patients with clinical stage 1B-3A non-small cell lung cancer (NSCLC) will receive neoadjuvant chemotherapy and cemiplimab every 3 weeks for 3 cycles with or without alirocumab every 4 weeks prior to surgery. Eligible patients will be randomized with equal allocation to two treatment groups. Permuted block randomization algorithm will...
Related Latest Advances
Brand Information
- To reduce the risk of major adverse cardiovascular (CV) events (coronary heart disease death, myocardial infarction, stroke, or unstable angina requiring hospitalization) in adults at increased risk for these events
- As an adjunct to diet and exercise to reduce low- density lipoprotein cholesterol (LDL-C) in:
- adults with hypercholesterolemia.
- adults and pediatric patients aged 8 years and older with heterozygous familial hypercholesterolemia (HeFH).
- adults with homozygous familial hypercholesterolemia (HoFH).
- 75 mg/mL single-dose pre-filled pen
- 150 mg/mL single-dose pre-filled pen
- Hypersensitivity Reactions
- Hypersensitivity reactions: Angioedema
- Influenza-like illness
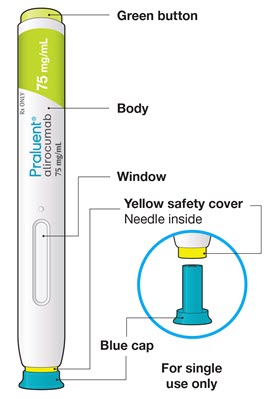
- the PRALUENT pen
- 1 alcohol wipe
- 1 cotton ball or gauze
- a sharps container or a puncture-resistant container (see
- Check that you have the correct product and the correct dose.
- Check the expiration date (EXP):

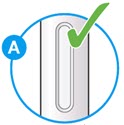
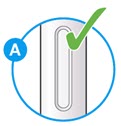
- Check the liquid is clear, colorless to pale yellow and free from particles (see
- You may see air bubbles. This is normal.
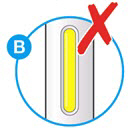
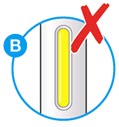
- Do not use if the window appears solid yellow (see Figure B).
- Do not use this medicine if the solution is discolored or cloudy, or if it contains visible flakes or particles.
- This is important for administering the entire dose and helps minimize discomfort.
- Take PRALUENT out of the refrigerator to warm up before using.
- Do not heat the pen, let it warm up on its own.
- Do not put the pen back in the refrigerator.
- Wash your hands with soap and water and dry with a towel.
- Clean skin in the injection area with an alcohol wipe.
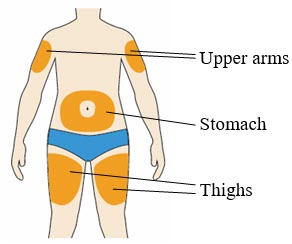
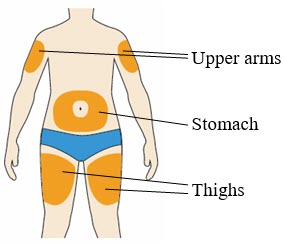
- You can inject into your (see
- You can stand or sit to give yourself an injection.
- Change (rotate) your injection site each time you give yourself an injection. If you need to use the same injection site, make sure it is not the same spot on the site you used last time.
- Do not inject into areas where the skin is tender, bruised, hard, or red. Do not inject PRALUENT into areas with visible veins, scars or stretch marks.
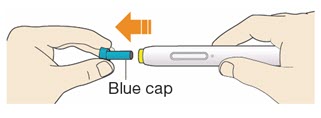
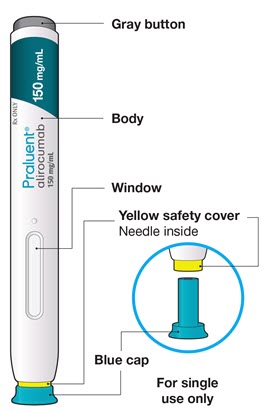
- Do not pull off the cap until you are ready to inject.
- Do not put the blue cap back on.
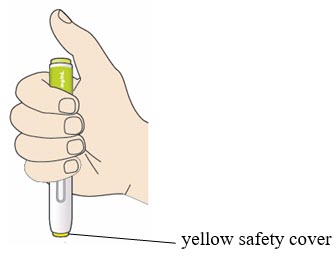
- Do not touch the yellow safety cover.
- Make sure you can see the window.
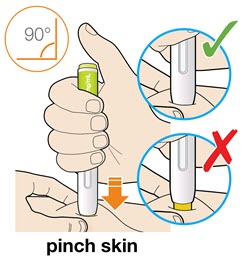
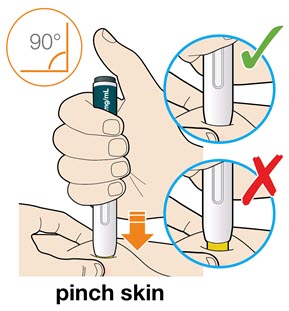
- For children younger than 12 years of age, pinching the skin before and during the injection is required.
- In adults and children aged 12 years and older, pinching of skin may be required to make the injection site firm.
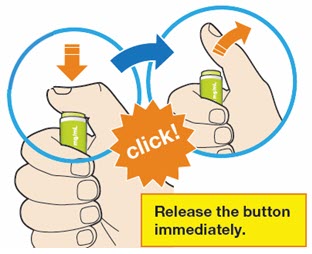
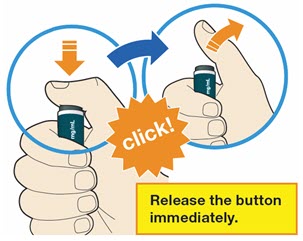
- Press and firmly hold the pen against your body until the yellow safety cover is no longer visible. The pen will not work if the yellow safety cover is not depressed fully.
- You will hear a click. Your injection has now started.
- The window will start to turn yellow.
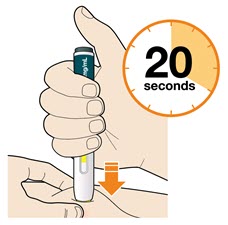
- The injection may take up to 20 seconds.
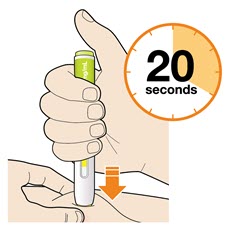
- Do not remove the pen until the entire window has turned yellow.
- Your injection is complete when the window has turned completely yellow, you may hear a second click.
- If the window does not turn completely yellow, call 1-844-772-5836 for help.

- Do not rub the skin after the injection.
- If you see any blood, press a cotton ball or gauze on the site until the bleeding stops.
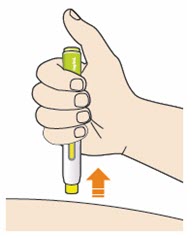
- Do not put the blue cap back on.
- Throw away pen and cap in a puncture-resistant container immediately after they have been used.
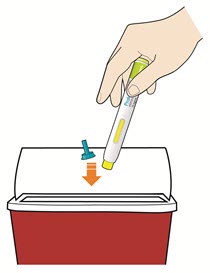
- Put your used pens in a FDA-cleared sharps disposal container right away after use.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
- the PRALUENT pen
- 1 alcohol wipe
- 1 cotton ball or gauze
- a sharps container or a puncture-resistant container (see
- Check that you have the correct product and the correct dose.
- Check the expiration date (EXP):

- Check the liquid is clear, colorless to pale yellow and free from particles (see
- You may see air bubbles. This is normal.
- Do not use if the window appears solid yellow (see Figure B).
- Do not use this medicine if the solution is discolored or cloudy, or if it contains visible flakes or particles.
- This is important for administering the entire dose and helps minimize discomfort.
- Take PRALUENT out of the refrigerator to warm up before using.
- Do not heat the pen, let it warm up on its own.
- Do not put the pen back in the refrigerator.
- Wash your hands with soap and water and dry with a towel.
- Clean skin in the injection area with an alcohol wipe.
- You can inject into your (see
- You can stand or sit to give yourself an injection.
- Change (rotate) your injection site each time you give yourself an injection. If you need to use the same injection site, make sure it is not the same spot on the site you used last time.
- Do not inject into areas where the skin is tender, bruised, hard, or red. Do not inject PRALUENT into areas with visible veins, scars or stretch marks.
- Do not pull off the cap until you are ready to inject.
- Do not put the blue cap back on.
- Do not touch the yellow safety cover.
- Make sure you can see the window.
- For children younger than 12 years of age, pinching the skin before and during the injection is required.
- In adults and children aged 12 years and older, pinching of skin may be required to make the injection site firm.
- Press and firmly hold the pen against your body until the yellow safety cover is no longer visible. The pen will not work if the yellow safety cover is not depressed fully.
- You will hear a click. Your injection has now started.
- The window will start to turn yellow.
- The injection may take up to 20 seconds.
- Do not remove the pen until the entire window has turned yellow.
- Your injection is complete when the window has turned completely yellow, you may hear a second click.
- If the window does not turn completely yellow, call 1-844-772-5836 for help.

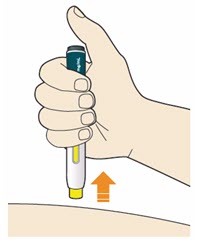
- Do not rub the skin after the injection.
- If you see any blood, press a cotton ball or gauze on the site until the bleeding stops.
- Do not put the blue cap back on.
- Throw away pen and cap in a puncture-resistant container immediately after they have been used.
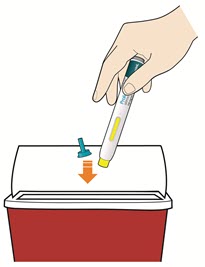
- Put your used pens in a FDA-cleared sharps disposal container right away after use.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
alirocumab
Injection
75 mg/mL
SANOFI
alirocumab
Injection
150 mg/mL
SANOFI
alirocumab
Injection
75 mg/mL
SANOFI
alirocumab
Injection
150 mg/mL
SANOFI