Brand Name
Guna-Diur
Generic Name
Amiloride
View Brand Information FDA approval date: January 22, 1986
Classification: Thiazide Diuretic
Form: Tablet, Solution
What is Guna-Diur (Amiloride)?
Amiloride HCl is indicated as adjunctive treatment with thiazide diuretics or other kaliureticdiuretic agents in congestive heart failure or hypertension to: help restore normal serum potassium levels in patients who develop hypokalemia on the kaliuretic diuretic. prevent development of hypokalemia in patients who would be exposed to particular risk if hypokalemia were to develop, e.g., digitalized patients or patients with significant cardiac arrhythmias. The use of potassium-conserving agents is often unnecessary in patients receiving diuretics for uncomplicated essential hypertension when such patients have a normal diet. Amiloride HCl has little additive diuretic or antihypertensive effect when added to a thiazide diuretic. Amiloride HCl should rarely be used alone. It has weak diuretic and antihypertensive effects. Used as single agents, potassium sparing diuretics, including amiloride HCl, result in an increased risk of hyperkalemia . Amiloride HCl should be used alone only when persistent hypokalemia has been documented and only with careful titration of the dose and close monitoring of serum electrolytes.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
Mechanisms of Diuretic Resistance in Heart Failure, Aim 2
Summary: Randomized placebo-controlled, double-blind, double-dummy, crossover design testing combinations placebo/placebo, bendroflumethiazide/placebo, amiloride/placebo, and bendroflumethiazide/amiloride added to bumetanide.
Beneficial Effect of Amiloride on Progression of Chronic Kidney Disease
Summary: This is a randomized, placebo-controlled, double-blinded crossover trial testing the effects of amiloride in patients with chronic kidney disease (CKD) and proteinuria. In CKD with proteinuria, there is aberrant filtration of serine proteases and complement precursors into the tubular lumen. The interaction of these factors leads to proinflammatory complement activation, which may promote inflamma...
Mechanisms of Diuretic Resistance in Heart Failure, Aim 3
Summary: Randomized double-blind placebo-controlled crossover study design
Related Latest Advances
Brand Information
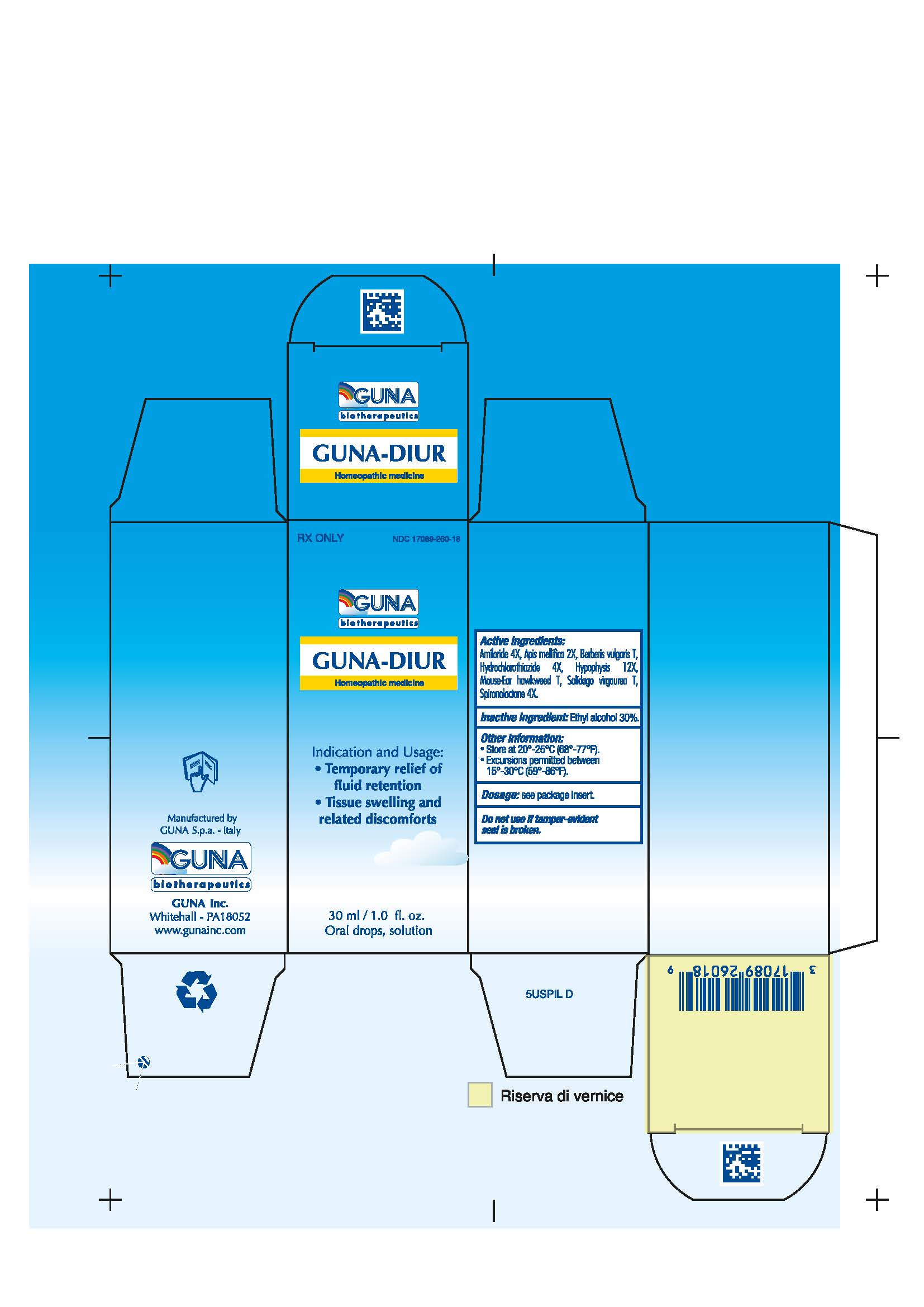
GUNA-DIUR (amiloride - apis mellifera - berberis vulgaris fruit - hieracium pilosella flowering top - hydrochlorothiazide - solidago virgaurea flowering top - spironolactone - sus scrofa pituitary gland -)
1INDICATIONS AND USAGE
1.1 Temporary relief of fluid retention
1.2 Tissue swelling and related disconforts
2DOSAGE AND ADMINISTRATION
Adults: 20 drops in a little water, 2 times per day for an avarage of two months.
Stop use and ask a doctor if symptoms persist more than 5 days.
Administration may very according to individual needs.
GUNA-DIUR may be used together with other homeopthic medicines.
3DOSAGE FORMS AND STRENGTHS
3.1. 30 ml Bottle dropper container contains:
4CONTRAINDICATIONS
4.1. There is no history of hypersensitivity to GUNA-DIUR. However, do not use if you are hypersensitive to any of the active ingredients of Guna-Diur.
5WARNINGS AND PRECAUTIONS
5.1. GUNA-DIUR is contraindicated in patients with anuria and in patients with a history of hypersensitivity to Spironolactone, Amiloride, or Hydrocholorthiazide.
5.3 Keep out of reach of children.
6ADVERSE REACTIONS
6.1. None known (see CONTRAINDICATIONS for hypersensitivity information).
7DRUG INTERACTIONS
7.1. None Known
8USE IN SPECIFIC POPULATIONS
8.1.
8.4. Geriatric use: No restrictions.
9DRUG ABUSE AND DEPENDENCE
9.1. No Known.
10OVERDOSAGE
10.1. No Known.
11DESCRIPTION
11. 1 GUNA-DIUR is a homeopathic medicine indicated for the temporary relief of fluid retention, tissue swelling and related disconforts.
12CLINICAL PHARMACOLOGY
12.1. GUNA-DIUR exerts a diuretic effect. This is based on homeopthica Materia Medica and homeopathic principles.
12.3. Pharmacokinetics
Not applicable to homeopthic medicinal products.
13NONCLINICAL TOXICOLOGY
13.1. Not available.
14CLINICAL STUDIES
14.1. GUNA-DIUR efficacy is not supported by clinical studies. It is based on homeopathic Materia Medica and scientific literature.
15REFERENCES
15.1. H.H. Reckeweg. Homeopathic Materia Medica. Aurelia Verlag.
16HOW SUPPLIED/STORAGE AND HANDLING
16.1. NDC 17089-260-18 Oral Solution/Drops 30 mL
17PATIENT COUNSELING INFORMATION
17.1. Patients should be informed about Homeopathy and the main differences with conventional clinical approaches.
18PACKAGE LABEL