Brand Name
Qbrexza
Generic Name
Glycopyrronium
View Brand Information FDA approval date: June 28, 2018
Classification: Cholinergic Muscarinic Antagonist
Form: Cloth
What is Qbrexza (Glycopyrronium)?
Qbrexza is indicated for topical treatment of primary axillary hyperhidrosis in adult and pediatric patients 9 years of age and older. Qbrexza is an anticholinergic indicated for topical treatment of primary axillary hyperhidrosis in adults and pediatric patients 9 years of age and older .
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
A Double-Blind, Multicentre, Randomized, Three-Period, Three-Treatment, Cross-Over Study to Evaluate the Effect of BGF MDI, BFF MDI, and Placebo MDI on Exercise Parameters in Participants With COPD (ATHLOS)
Summary: This study will investigate the effect of Budesonide, Glycopyrronium, and Formoterol Fumarate (BGF) metered dose inhaler (MDI) compared with Placebo MDI, and Budesonide and Formoterol Fumarate (BFF) MDI on isotime inspiratory capacity (IC) and exercise endurance time.
A Randomized, Double-blind, Parallel Group, Multi-center, Phase III Study to Assess the Efficacy of Budesonide, Glycopyrronium, and Formoterol Fumarate Metered Dose Inhaler Relative to Glycopyrronium and Formoterol Fumarate MDI on Cardiopulmonary Outcomes in Chronic Obstructive Pulmonary Disease (THARROS)
Summary: This study will evaluate the effect of triple ICS/LAMA/LABA therapy with BGF MDI 320/14.4/9.6 μg on cardiopulmonary outcomes relative to LAMA/LABA therapy with GFF MDI 14.4/9.6 μg in a population with COPD and elevated cardiopulmonary risk.
Chronicling the COPD Patient Journey and Change in Impact of a Single Inhaler Combination Therapy on COPD Symptoms, Quality of Life and Exacerbations Following Initiation of Budenoside/Glycopyrronium/Formoterol [BGF](CHRONICLES COPD)
Summary: The CHRONICLES study will investigate the change in clinical and patient reported outcomes after six-months of treatment with Budenoside/Glycopyrronium/Formoterol \[BGF\] in a real-world setting.
Related Latest Advances
Brand Information
Qbrexza (glycopyrronium)
1INDICATIONS AND USAGE
Qbrexza is indicated for topical treatment of primary axillary hyperhidrosis in adult and pediatric patients 9 years of age and older.
2DOSAGE AND ADMINISTRATION
For topical use only.
Qbrexza is for topical use in the underarm area only and not for use in other body areas.
Qbrexza is administered by a single-use pre-moistened cloth packaged in individual pouches. Qbrexza should be applied to clean dry skin on the underarm areas only. Qbrexza should not be used more frequently than once every 24 hours.
Tear open the pouch and pull out the cloth, unfold the cloth, and wipe it across one entire underarm once. Using the same cloth, wipe the other underarm once. A single cloth should be used to apply Qbrexza to both underarms.
After applying Qbrexza, discard the cloth in the household trash out of reach of children and others. Wash hands immediately with soap and water after applying and discarding the Qbrexza cloth. Qbrexza may cause temporary dilation of the pupils and blurred vision if it comes in contact with the eyes. Avoid transfer of Qbrexza to the periocular area
Do not apply Qbrexza to broken skin. Avoid using Qbrexza with occlusive dressings.
3DOSAGE FORMS AND STRENGTHS
Cloth: A single-use cloth pre-moistened with 2.4% glycopyrronium solution
4CONTRAINDICATIONS
Qbrexza is contraindicated in patients with medical conditions that can be exacerbated by the anticholinergic effect of Qbrexza (e.g., glaucoma, paralytic ileus, unstable cardiovascular status in acute hemorrhage, severe ulcerative colitis, toxic megacolon complicating ulcerative colitis, myasthenia gravis, Sjogren’s syndrome).
5ADVERSE REACTIONS
The following adverse reactions are described in greater detail in other sections
• New or Worsening Urinary Retention
5.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In two double-blind, vehicle-controlled clinical trials (Trial 1 [NCT02530281] and Trial 2 [NCT02530294]) of 459 subjects treated with Qbrexza once daily and 232 treated with vehicle, subjects were 9 to 76 years of age, 47% male, and the percentages of White, Black (including African Americans), and Asian subjects were 82%, 12%, and 1%, respectively.
Table 1 summarizes the most frequent adverse reactions (≥2%) in subjects with primary axillary hyperhidrosis treated with Qbrexza.
Table 2 shows the most frequently reported local skin reactions, which were relatively common in both the Qbrexza and vehicle groups.
In an open-label safety trial (NCT02553798), 564 subjects were treated for up to an additional 44 weeks after completing Trial 1 or Trial 2. Adverse reactions occurring at a frequency ≥2.0% were: dry mouth (16.9%), vision blurred (6.7%), nasopharyngitis (5.8%), mydriasis (5.3%), urinary hesitation (4.2%), nasal dryness (3.6%), dry eye (2.9%), pharyngitis (2.2%), and application site reactions (pain [6.4%], dermatitis [3.8%], pruritus [3.8%], rash [3.8%], erythema [2.4%]).
5.2Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Qbrexza. Because the reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to Qbrexza exposure.
•
6OVERDOSAGE
Because glycopyrronium is a quaternary amine which does not easily cross the blood-brain barrier, symptoms of glycopyrronium overdosage are generally more peripheral in nature rather than central compared to other anticholinergic agents. Associated signs and symptoms related to excessive anticholinergic activity may include flushing, hyperthermia, tachycardia, ileus, urinary retention, loss of ocular accommodation and light sensitivity due to mydriasis.
In the case of overdose when symptoms are severe or life threatening, therapy may include:
- Managing per standard of care any acute conditions such as hyperthermia, coma, and/or seizures, as applicable, and managing any myoclonic or choreoathetoid movements which may lead to rhabdomyolysis in some cases of anticholinergic overdosage
- Managing severe urinary retention with catheterization if not spontaneously reversed within several hours
- Providing cardiovascular support and/or controlling arrhythmias
- Maintaining an open airway, providing ventilation as necessary
- Administering a quaternary ammonium anticholinesterase such as neostigmine to help alleviate severe and/or life threatening peripheral anticholinergic effects.
Topical overdosing of Qbrexza could result in an increased incidence or severity of local skin reactions. Administration of Qbrexza under occlusive conditions may result in an increase in anticholinergic effects, including dry mouth and urinary hesitation.
7DESCRIPTION
Qbrexza (glycopyrronium) cloth, 2.4% is an anticholinergic drug available as a clear, colorless to pale yellow solution on a single-use pre-moistened cloth (an absorbent polypropylene pad) packaged in a pouch for topical administration. Each pouch contains 105 mg glycopyrronium tosylate, equivalent to 66 mg of glycopyrronium. The inactive ingredients are citric acid, dehydrated alcohol, purified water, and sodium citrate.
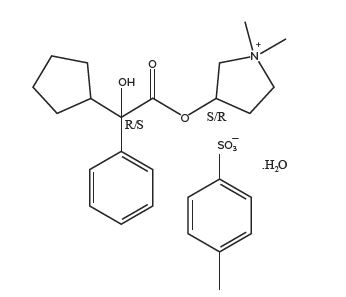
Glycopyrronium tosylate is chemically described as pyrrolidinium, 3-[(2-cyclopentyl-2-hydroxy-2-phenylacetyl)oxy]-1,1-dimethyl-, 4-methylbenzensulfonate, hydrate (1:1:1) with an empirical formula of C
8PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (
New or Worsening Urinary Retention
Instruct patients to be alert for signs and symptoms of urinary retention (e.g., difficulty passing urine, distended bladder). Instruct patients to discontinue use and consult a physician immediately should any of these signs or symptoms develop.
Control of Body Temperature (Risk of Overheating or Heat Illness)
In the presence of high ambient temperature, heat illness due to decreased sweating can occur with the use of anticholinergic drugs such as Qbrexza. Advise patients using Qbrexza to watch for generalized lack of sweating when in hot or very warm environmental temperatures and to avoid use if not sweating under these conditions.
Operating Machinery or an Automobile
Transient blurred vision may occur with Qbrexza. If this occurs, instruct patients to contact their healthcare provider, discontinue use of Qbrexza and avoid operating a motor vehicle or other machinery, or performing hazardous work until symptoms resolve.
Instructions for Administering Qbrexza
It is important for patients to understand how to correctly apply Qbrexza (see
- Instruct patients to use one cloth to apply Qbrexza to both axillae by wiping the cloth across one underarm, ONE TIME.
- Using the same cloth, apply the medication to the other underarm, ONE TIME.
- After applying Qbrexza, discard the cloth in the household trash out of reach of children and others.
- Wash your hands with soap and water right away after you apply Qbrexza and have thrown away the cloth.
- Inform patients to avoid touching the periocular area. The Qbrexza that is still on your hands can cause you to have temporary pupil dilation and blurred vision if you touch your eyes.
- Remind patients not to apply Qbrexza to other body areas or to broken skin. Instruct patients to avoid using Qbrexza with occlusive dressings.
- Qbrexza is flammable; avoid use near heat or flame.
Manufactured for:
Journey Medical Corporation
Version 2, October 2022
9PRINCIPAL DISPLAY PANEL - NDC: 69489-411-01 - 1-Count Pouch Label
NDC 69489-411-01
Cloth
Qbrexza
(glycopyrronium) cloth
2.4%
(glycopyrronium) cloth
2.4%
For Topical Use Only
Each pouch contains 1 cloth with 2.8 grams of solution
Avoid touching eyes.
immediately after discarding the used cloth.

10PRINCIPAL DISPLAY PANEL - NDC: 69489-411-30 - 30-Count Carton Label
NDC 69489-411-30
Rx Only
Qbrexza
(glycopyrronium) cloth
2.4%
(glycopyrronium) cloth
2.4%
For Topical Use Only
30 Single-use Cloths
www.Qbrexza.com
Avoid touching eyes. Wash hands with soap and water
immediately after discarding the used cloth.