Brand Name
Sofdra
Generic Name
Sofpironium
View Brand Information FDA approval date: August 06, 2024
Form: Gel
What is Sofdra (Sofpironium)?
SOFDRA is indicated for the treatment of primary axillary hyperhidrosis in adults and pediatric patients 9 years of age and older. SOFDRA is an anticholinergic indicated for the treatment of primary axillary hyperhidrosis in adults and pediatric patients 9 years of age and older .
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Sofdra (sofpironium bromide)
1INDICATIONS AND USAGE
SOFDRA is indicated for the treatment of primary axillary hyperhidrosis in adults and pediatric patients 9 years of age and older.
2DOSAGE AND ADMINISTRATION
- Do not shave armpits at least 8 hours before applying SOFDRA.
- Do not shower at least 30 minutes before applying SOFDRA.
- Apply SOFDRA to clean, dry skin once a day at bedtime.
- Apply a single pump actuation to the top of the supplied applicator. Spread the entire amount to cover 1 underarm. Apply a separate, single pump actuation to the top of the supplied applicator. Apply the entire amount to the second underarm. Allow to dry completely (5 minutes) before putting on clothing.
- Wash hands immediately with soap.
- For topical use only.
- Avoid fire, flame, and smoking during and immediately following application.
- Do not shower or wash underarms for at least 8 hours after application.
- Do not touch underarms after applying SOFDRA.
- Do not use more than once daily.
- Avoid transfer of SOFDRA to the periocular area
- Do not apply SOFDRA to broken skin.
- Avoid using SOFDRA with occlusive dressings.
3DOSAGE FORMS AND STRENGTHS
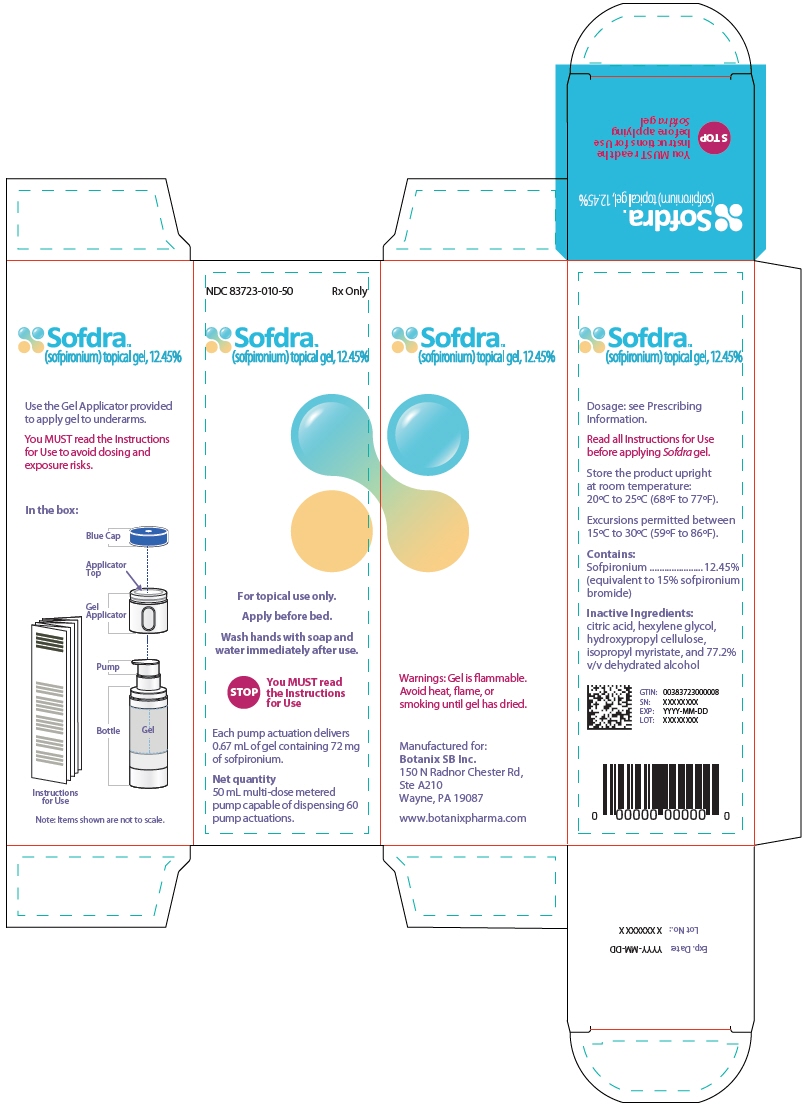
Topical gel: 12.45% (w/w) of sofpironium in a 50 mL bottle with a metered dose pump and applicator. One full pump delivers 72 mg sofpironium in 0.67 mL of gel.
4CONTRAINDICATIONS
SOFDRA is contraindicated in patients with medical conditions that can be exacerbated by the anticholinergic effect of sofpironium bromide (e.g., glaucoma, paralytic ileus, unstable cardiovascular status in acute hemorrhage, severe ulcerative colitis, toxic megacolon complicating ulcerative colitis, myasthenia gravis, Sjögren's syndrome).
5ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
Urinary Retention
5.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In two double-blind, vehicle controlled clinical trials (CARDIGAN 1 and CARDIGAN 2) of 700 subjects 10 to 76 years of age (353 subjects treated with SOFDRA and 347 subjects treated with vehicle), 44% of subjects were male, 79% were White, 21% were Black, and 1% were Asian. A total of 618 subjects completed at least 6 weeks of treatment, including 307 subjects treated with SOFDRA and 311 subjects treated with vehicle.
Table 1 summarizes the most frequent adverse reactions (≥2%) in subjects with primary axillary hyperhidrosis treated with SOFDRA.
Table 2 shows the local skin reactions reported ≥2%, which occurred more commonly in the SOFDRA group.
In an open-label, long-term safety trial (ARGYLE), 197 subjects were treated for 48 weeks with SOFDRA. Adverse reactions occurring at a frequency ≥2% were vision blurred (19%), dry mouth (17%), application site pruritus (15%), application site pain (15%), application site dermatitis (11%), application site erythema (8%), application site irritation (6%), mydriasis (5%), application site rash (4%), upper respiratory tract infection (4%), dry eye (4%), urinary retention (4%), application site exfoliation (3%), application site folliculitis (3%), hypertension (3%), application site dryness (2%), viral upper respiratory tract infection (2%), influenza (2%), and headache (2%).
6OVERDOSAGE
In case of an overdose, remove the topically applied product with soap and water, and treat the symptoms and signs attributed to the overdose symptomatically. Consider contacting the Poison Center at 1-800-222-1222 for the latest recommendations.
7DESCRIPTION
SOFDRA (sofpironium) topical gel is an anticholinergic drug. Sofpironium bromide drug substance is a white to off white powder with the chemical name 3'(R)-[2(R) cyclopentylphenylhydroxy-acetoxy]-1'-methyl-1'-ethoxycarbonylmethyl-pyrrolidinium bromide, very soluble in chloroform and freely soluble in water, ethanol, acetonitrile, and methanol, molecular formula of C

SOFDRA is a clear to translucent, colorless to pale yellow viscous gel containing 12.45% (w/w) sofpironium (equivalent to 15% sofpironium bromide) in an airless bottle sealed with a multi-dose metered pump. Each pump delivers 72 mg of sofpironium (equivalent to 87 mg of sofpironium bromide) in 0.67 mL of gel. The inactive ingredients are citric acid, 77.2% v/v dehydrated alcohol, hexylene glycol, hydroxypropyl cellulose, and isopropyl myristate.
8CLINICAL STUDIES
Two randomized, vehicle-controlled multicenter trials, CARDIGAN 1 (NCT03836287) and CARDIGAN 2 (NCT03948646), enrolled a total of 701 subjects 10 years of age or older with primary axillary hyperhidrosis. All subjects were to have symptoms of axillary hyperhidrosis for at least 6 months' duration, produce at least 50 mg of sweat in each axilla (underarm) with a combined total of at least 150 mg over a 5-minute period, and have a Hyperhidrosis Disease Severity Measure-Axillary, 7-item scale score (HDSM-Ax-7) ≥3.
In the trials, 56% of subjects were female, 78% were White, and 20% were Black or African American; for ethnicity, 31% identified as Hispanic or Latino. Fewer than 1% of subjects were less than 12 years of age, 7% were 12 to 17 years of age, 91% were 18 to 64 years of age, and 1% were 65 years of age or older.
Subjects 12 years of age and older were asked to rate their underarm sweating severity and frequency since waking on the previous day ("since you woke up yesterday") on the 11-item HDSM-Ax Adult version instrument. The HDSM-Ax-7 scale score was calculated by taking an average of 7 items, where the scale score ranges from 0 to 4 with a higher score representing greater underarm sweating severity. The mean HDSM-Ax-7 scale score at Baseline was 3.5 in CARDIGAN 1, and 3.6 in CARDIGAN 2. The median gravimetric sweat production (GSP) over 5 minutes at Baseline was 214.1 mg in the SOFDRA arm and 228.6 mg in the vehicle arm in CARDIGAN 1, and 207.7 mg in the SOFDRA arm and 231.1 mg in the vehicle arm in CARDIGAN 2.
Subjects were randomized to receive either SOFDRA or vehicle applied once daily at bedtime to each axilla. The co-primary endpoints were the proportion of subjects having at least a 2-point improvement in the HDSM-Ax-7 scale score from Baseline to Day 43, and the change in GSP from Baseline to Day 43.
The results of CARDIGAN 1 and CARDIGAN 2 are presented in Table 5.
9PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
10INSTRUCTIONS FOR USE
SOFDRA™
(sof-drah)
(sofpironium)
topical gel, 12.45%
(sof-drah)
(sofpironium)
topical gel, 12.45%
These Instructions for Use contain information on how to use SOFDRA.

Read the Instructions for Use before you start using SOFDRA and each time you get a refill.
This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
If you have questions, ask your pharmacist or healthcare provider.
Prepare to Apply SOFDRA
1 Check if you are ready to apply SOFDRA
1.1Apply SOFDRA before bedtime (Figure B).
1.2 Before applying SOFDRA make sure that you:
2 Gather supplies
2.1 Gather the following supplies.
- SOFDRA(Figure C)
- Clean towel(Figure D)
3 Check the expiration date
3.1 Check the expiration date("EXP") on the Bottle label to make sure it has not passed (Figure E).
4 Remove Blue Cap and Applicator from Bottle
4.1 Pullthe Blue Cap straight offthe Bottle and set it aside (Figure F).
4.2 Pullthe gel Applicator straight offthe Bottle and set it aside (Figure G).
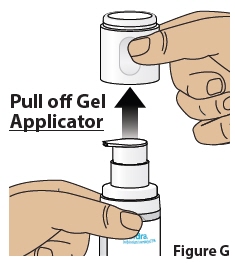

5 Prime the pump(First time use only)
6 Prepare underarms
Apply 1 Pump of SOFDRA to Each Armpit
7 Apply 1 pump of SOFDRA gel to
8 Apply 1 pump of SOFDRA gel to
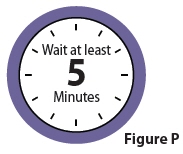
9 Let SOFDRA gel dry
9.1 Let SOFDRA gel dry for 5 minutesafter it is applied (Figure P). Keep clothing off the area while it dries.
Note:It is okay to put your arms down.
Note:It is okay to put your arms down.
What to do After Applying SOFDRA
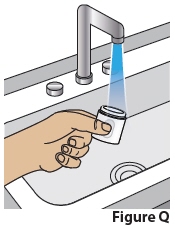
10 Rinse and dry Applicator
10.1 Rinse off the Applicator with water(Figure Q).
Avoidtouching the gel with your hands or fingers.
Avoidtouching the gel with your hands or fingers.
10.2 Pat the Applicator dry(Figure R).

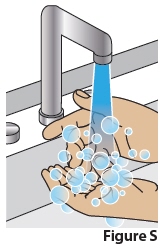
11 Wash hands
11.1 Always wash your hands with soap and water right away after applying SOFDRA gelto avoid spreading any gel residue to any other parts of your body (Figure S).
12 Reassemble and store SOFDRA after use
13 Keep SOFDRA on skin for at least 8 hours
13.1 Wait at least 8 hoursafter applying SOFDRA before you shower or wash your underarms (Figure W).
11PRINCIPAL DISPLAY PANEL - 50 mL Bottle Carton
NDC 83723-010-50
Sofdra
For topical use only.
Apply before bed.
Wash hands with soap and
STOP
Each pump actuation delivers
Net quantity