Rayaldee
What is Rayaldee (Calcifediol)?
Approved To Treat
Related Clinical Trials
Summary: Patients with hypovitaminosis D are randomized into three arms of treatment: Group A: Calcifediol 0,266mg each month Group B: Cholecalciferol 25000UI each 15 days Group C: Calcifediol 4 drops per day. Serum levels of vitamin D are dosed after one month of treatment
Summary: Trauma has been an important global public health issue. Yet it is the sixth cause of death in Taiwan, trauma brings great negative impact to national productivity since it presents specifically as the leading cause of death for the population aged below 40 years. According to the national databank from Formosa Association for the Surgery of Trauma, mortality rate in critically traumatic patients ...
Summary: There are no clear international guidelines for dosing vitamin D based on deficiency severity. Therefore, a new clinical trial is needed to evaluate the benefits of early vitamin D supplementation in maintaining sufficient levels for critically ill patients. The investigators conducted a multicenter clinical trial in Taiwan focusing on vitamin D and critically ill patients. 240 patients with low c...
Related Latest Advances
Brand Information
- blue oval soft capsules imprinted with “O” in white ink
- White two-piece banded hard capsules imprinted with ”O” and “30” in blue ink
- Hypercalcemia [
- Adynamic Bone Disease [
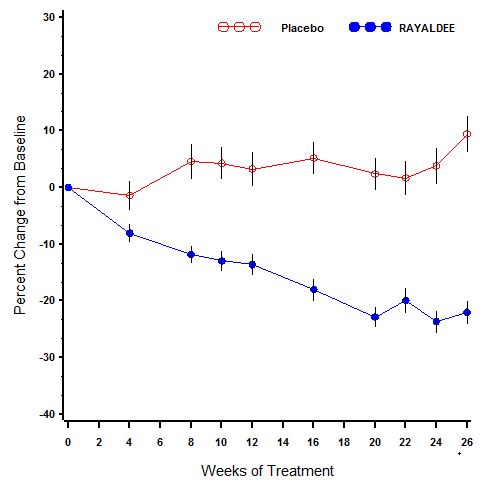
- Inform patients to take RAYALDEE at bedtime and to swallow the capsules whole.
- Inform patients if they miss a dose, to take RAYALDEE at the next scheduled time. Do not take an extra dose to make up for the missed dose.
- Inform patients that they will need routine monitoring of laboratory parameters such as calcium, iPTH and total 25-hydroxyvitamin D while taking RAYALDEE.
- Advise patients to contact a health care provider if they develop symptoms of elevated calcium (e.g., feeling tired, having difficulty thinking clearly, loss of appetite, nausea, vomiting, constipation, increased thirst, increased urination or weight loss).
- Advise patients to inform their physician of all use of medications, including prescription and nonprescription drugs, supplements and herbal preparations, and of any changes in medical condition. Patients should also be advised to inform their physicians, when receiving a newly prescribed medication, that they are taking RAYALDEE.
- Inform lactating women about the need to monitor infants exposed to RAYALDEE through breast milk for signs of hypercalcemia to include seizures, vomiting, constipation and weight loss [


30 mcg


