Uplizna
What is Uplizna (Inebilizumab)?
Related Clinical Trials
Summary: A Phase 2, open-label, multicenter study to evaluate the pharmacokinetics (PK), pharmacodynamics (PD), and safety of inebilizumab in eligible pediatric participants 2 to \< 18 years of age with recently active neuromyelitis optica spectrum disorder (NMOSD) who are seropositive for autoantibodies against aquaporin-4 (AQP4-immunoglobulin \[Ig\]G).
Summary: The primary objectives of this study are to characterize the pharmacokinetics (PK) and pharmacodynamics (PD) of inebilizumab administered in pediatric participants with gMG, and to assess the safety and tolerability of inebilizumab administered in pediatric participants with gMG.
Summary: This is an observational study to monitor female participants exposed to UPLIZNA during pregnancy. This study requires voluntary reporting of pregnancies in female participants with NMOSD exposed to UPLIZNA during pregnancy or within 6 months preceding conception. Pregnancy-related data, potential confounding factors and information related to pregnancy outcome will be collected. The schedule of o...
Related Latest Advances
Brand Information
- A history of a life-threatening infusion reaction to UPLIZNA
- Active hepatitis B infection
- Active or untreated latent tuberculosis
- Infusion Reactions
- Infections
- Reduction in Immunoglobulins
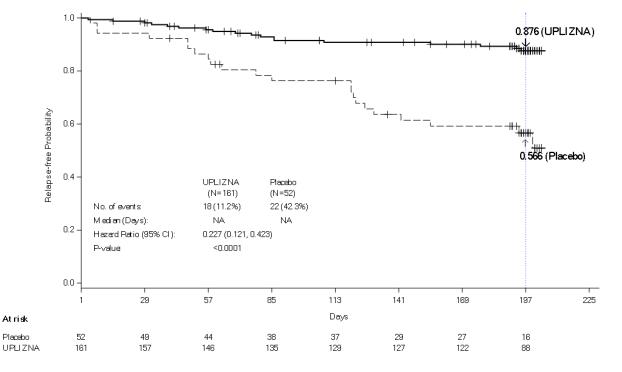
- A history of one or more relapses that required rescue therapy within the year prior to screening, or 2 or more relapses that required rescue therapy in 2 years prior to screening.
- Expanded Disability Status Scale (EDSS) score of 7.5 or less. Patients with an EDSS score of 8.0 were eligible if they were deemed capable of participating.
- Patients were excluded if previously treated with immunosuppressant therapies within an interval specified for each such therapy.