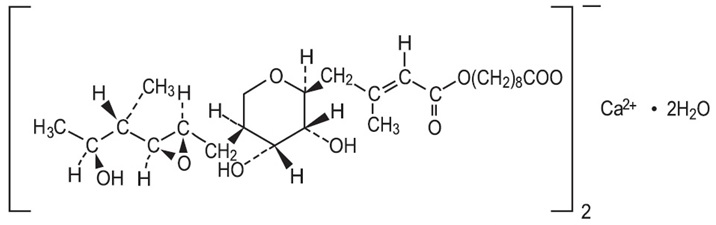
Mupirocin
What is Pirnuo (Mupirocin)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: This protocol aims to evaluate how NMT affects pediatric nasal microbiome diversity following intranasal mupirocin treatment
Summary: The goal of this clinical trial is to learn if reducing bacterial load on the skin and nostrils with topical antibacterial soap and ointment, respectively, reduces rate of infection in surgical sites on lower leg wounds left open to heal in adults undergoing skin cancer surgery. The main question it aims to answer is: Does Hibiclens antibacterial skin cleanser and mupirocin antibacterial ointment ...
Summary: The DECREASE SSI Trial (Decolonization to Reduce After-Surgery Events of Surgical Site Infection) is a two-arm multi-center individual placebo-controlled randomized (2,700 participants randomized 1:1) clinical trial to reduce post-discharge surgical site infection following open colon or small bowel surgery by comparing chlorhexidine bathing plus nasal mupirocin in the 30 days following discharge ...
Related Latest Advances
Brand Information
- For Topical Use Only.
- Apply a small amount of PIRNUO cream, with a cotton swab or gauze pad, to the affected area 3 times daily for 10 days.
- Cover the treated area with gauze dressing if desired.
- Re-evaluate patients not showing a clinical response within 3 to 5 days.
- PIRNUO cream is not for intranasal, ophthalmic, or other mucosal use
- Do not apply PIRNUO cream concurrently with any other lotions, creams, or ointments
- Severe Allergic Reactions
- Eye Irritation
- Local Irritation
- Clostridium difficile-Associated Diarrhea [see Warnings and Precautions (
- Clinical and Laboratory Standards Institute (CLSI).
- Patel J, Gorwitz RJ, et al. Mupirocin Resistance.
- Clinical and Laboratory Standards Institute (CLSI).
- Clinical and Laboratory Standards Institute (CLSI).
- Finlay JE, Miller LA, Poupard JA. Interpretive criteria for testing susceptibility of staphylococci to mupirocin.
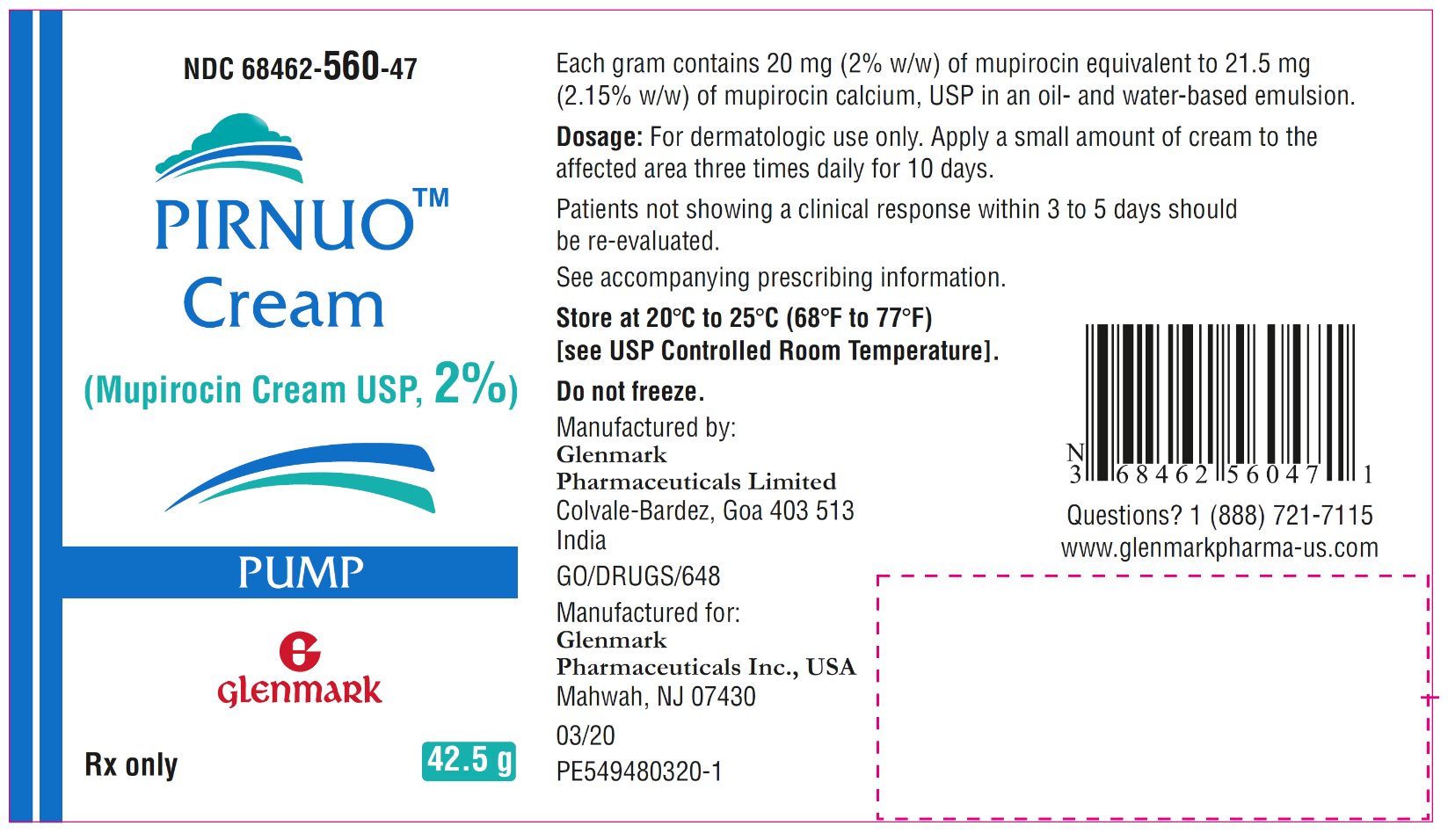
- NDC 68462-560-47 42.5-gram pump (1 pump per carton)
- Use PIRNUO cream only as directed by the healthcare provider. It is for external use only. Avoid contact of PIRNUO cream with the eyes. If PIRNUO cream gets in the eyes, rinse thoroughly with water.
- Do not use PIRNUO in cream in the nose.
- Wash your hands before and after applying PIRNUO cream.
- Use a gauze pad or cotton swab to apply a small amount of PIRNUO cream to the affected area. The treated area may be covered by gauze dressing if desired.
- Report to the healthcare provider any signs of local adverse reactions. PIRNUO cream should be stopped and the healthcare provider contacted if irritation, severe itching, or rash occurs.
- Report to the healthcare provider or go to the nearest emergency room if severe allergic reactions, such as swelling of the lips, face, or tongue, or wheezing occur
- If no improvement is seen in 3 to 5 days, contact the healthcare provider.

- you are allergic to mupirocin or any of the ingredients in PIRNUO cream. See the end of this Patient Information leaflet for a complete list of the ingredients in PIRNUO cream.
- are pregnant or plan to become pregnant. It is not known if PIRNUO cream will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if PIRNUO cream passes into your breast milk. You and your healthcare provider should decide if you can use PIRNUO cream while breastfeeding.
- PIRNUO cream is for use on the skin (topical). Do not get PIRNUO cream in your eyes, nose, mouth, or vagina (mucosal surfaces).
- Use PIRNUO cream exactly as your healthcare provider tells you to use it.
- Apply a small amount of PIRNUO cream, with a cotton swab or gauze pad, to the affected area 3 times each day. Apply PIRNUO cream for 10 days.
- It is important that you take the full course of PIRNUO cream. Do not stop early because your symptoms may disappear before the infection is fully cleared.
- Wash your hands
- After applying PIRNUO cream, you may cover the treated area with a clean gauze pad, unless your healthcare provider has told you to leave it uncovered.
- Talk to your healthcare provider if your skin does not improve after 3 to 5 days of treatment with PIRNUO cream.
- If you are breastfeeding and use PIRNUO cream on your breast or nipple, wash the area well before breastfeeding your child.
- severe allergic reactions. Stop using PIRNUO cream and call your healthcare provider or go to the nearest emergency room right away if you have any of the following signs or symptoms of a severe allergic reaction:
- eye irritation. Do not get PIRNUO cream in your eyes. If PIRNUO cream gets in your eyes, rinse your eyes well with water.
- irritation in the area PIRNUO cream is used. A rash may occur after using PIRNUO cream and can be severe. Stop using PIRNUO cream and call your healthcare provider if you develop an irritation, severe itching, or a rash while using PIRNUO cream.
- a type of diarrhea called CDAD may happen in people who use or have used medicine to treat bacterial infections. The severity of CDAD can range from mild diarrhea to severe diarrhea that may cause death (fatal colitis). Call your healthcare provider or go to the nearest emergency room right away if you have diarrhea while using or after you stop using PIRNUO cream.
- headache
- rash
- nausea
- Store PIRNUO cream at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not freeze PIRNUO cream.
- Keep PIRNUO cream and all medicines out of the reach of children.

- This Patient Information has been approved by the U.S. Food and Drug Administration Revised: March 2020