Brand Name
Luzu
Generic Name
Luliconazole
View Brand Information FDA approval date: November 14, 2013
Classification: Azole Antifungal
Form: Cream
What is Luzu (Luliconazole)?
LUZU Cream, 1% is indicated for the topical treatment of interdigital tinea pedis, tinea cruris, and tinea corporis caused by the organisms Trichophyton rubrum and Epidermophyton floccosum. LUZU Cream, 1% is an azole antifungal indicated for the topical treatment of interdigital tinea pedis, tinea cruris, and tinea corporis caused by the organisms Trichophyton rubrum and Epidermophyton floccosum.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Luzu (LULICONAZOLE)
1INDICATIONS AND USAGE
LUZU (luliconazole) Cream, 1% is indicated for the topical treatment of interdigital tinea pedis, tinea cruris, and tinea corporis caused by the organisms
2DOSAGE AND ADMINISTRATION
For topical use only. LUZU Cream, 1% is not for ophthalmic, oral, or intravaginal use.
- When treating interdigital tinea pedis, a thin layer of LUZU Cream, 1% should be applied to the affected area and approximately 1 inch of the immediate surrounding area(s) once daily for 2 weeks.
- When treating tinea cruris or tinea corporis, LUZU Cream, 1% should be applied to the affected area and approximately 1 inch of the immediate surrounding area(s) once daily for 1 week.
3DOSAGE FORMS AND STRENGTHS
Cream, 1%. Each gram of LUZU Cream, 1% contains 10 mg of luliconazole in a white cream base.
4CONTRAINDICATIONS
None.
5DRUG INTERACTIONS
An in vivo study in adult subjects with moderate to severe interdigital tinea pedis and tinea cruris showed that LUZU Cream, 1% is mostly a weak inhibitor of CYP2C19. In a separate trial in adolescent subjects with tinea cruris, in vivo blood levels of LUZU Cream, 1%, were seen to approach those levels sufficient to show moderate inhibition of CYP2C19
6DESCRIPTION
LUZU (luliconazole) Cream, 1% contains 1% luliconazole, an azole antifungal agent, in a white cream for topical application.
Luliconazole is (2E)-2-[(4R)-4-(2,4-dichlorophenyl)-1,3-dithiolan-2-ylidene]-2-imidazol-1-ylacetonitrile. Its structural formula is:
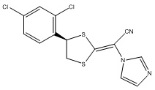
The molecular formula is C
LUZU Cream, 1% contains 10 mg of luliconazole per gram of cream in a vehicle consisting of benzyl alcohol, butylated hydroxytoluene, cetostearyl alcohol, isopropyl myristate, medium-chain triglycerides, methylparaben, polysorbate 60, propylene glycol, purified water, and sorbitan monostearate.
7HOW SUPPLIED/STORAGE AND HANDLING
LUZU (luliconazole) Cream, 1% is a white cream supplied in tubes as follows:
60 g NDC 99207-850-60
8PATIENT COUNSELING INFORMATION
Advise the patient to read the
- Inform patients that LUZU Cream, 1% is for topical use only. LUZU Cream, 1% is not intended for intravaginal or ophthalmic use.
Distributed by:
Bausch Health US, LLC
Bridgewater, NJ 08807 USA
Bausch Health US, LLC
Bridgewater, NJ 08807 USA
Manufactured by:
Bausch Health Companies Inc.
Laval, Quebec H7L 4A8, Canada
Bausch Health Companies Inc.
Laval, Quebec H7L 4A8, Canada
U.S. Patent Numbers: 8,980,931; 9,012,484; 9,199,977 and 9,453,006
LUZU is a trademark of Bausch Health Companies Inc. or its affiliates.
© 2020 Bausch Health Companies Inc. or its affiliates
9438803
9PATIENT INFORMATION
LUZU
(luliconazole) Cream, 1%
(luliconazole) Cream, 1%
What is LUZU Cream?
LUZU Cream is a prescription medicine used on the skin (topical) to treat fungal infections in people with athlete’s foot that is between the toes, jock itch, and ringworm.
Before using LUZU Cream, tell your doctor about all of your medical conditions, including if you:
- are pregnant or plan to become pregnant. It is not known if LUZU Cream will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if LUZU Cream passes into your breast milk.
Tell your doctor about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use LUZU Cream?
- Use LUZU Cream exactly as your doctor tells you to use it.
- If you have athlete’s foot between the toes, apply a thin layer of LUZU Cream to the affected skin areas and to about 1 inch of the surrounding skin 1 time a day for 2 weeks.
- If you have jock itch or ringworm, apply LUZU Cream to the affected skin areas and to about 1 inch of the surrounding skin 1 time a day for 1 week.
- Wash your hands after you apply LUZU Cream.
What are the possible side effects of LUZU Cream?
LUZU Cream may cause skin reactions at the treatment site. Skin irritation may happen with LUZU Cream. Tell your doctor if you have any skin reactions on the areas of your skin treated with LUZU Cream.
These are not all the possible side effects of LUZU Cream.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store LUZU Cream?
- Store LUZU Cream at room temperature between 68°F to 77°F (20°C to 25°C).
Keep LUZU Cream and all medicines out of the reach of children.
General information about the safe and effective use of LUZU Cream
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use LUZU Cream for a condition for which it was not prescribed. Do not give LUZU Cream to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or doctor for information about LUZU Cream that is written for health professionals.
What are the ingredients in LUZU Cream?
Active ingredient: luliconazole
Inactive ingredients: benzyl alcohol, butylated hydroxytoluene, cetostearyl alcohol, isopropyl myristate, medium-chain triglycerides, methylparaben, polysorbate 60, propylene glycol, purified water, sorbitan monostearate.
Distributed by:
Bausch Health US, LLC
Bridgewater, NJ 08807 USA
Bausch Health US, LLC
Bridgewater, NJ 08807 USA
Manufactured by:
Bausch Health Companies Inc.
Laval, Quebec H7L 4A8, Canada
Bausch Health Companies Inc.
Laval, Quebec H7L 4A8, Canada
U.S. Patent Numbers: 8,980,931; 9,012,484; 9,199,977 and 9,453,006
LUZU is a trademark of Bausch Health Companies Inc. or its affiliates.
© 2020 Bausch Health Companies Inc. or its affiliates
For more information, call 1-800-321-4576.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: 04/2020
