Photrexa Cross-linking
What is Photrexa Cross-linking (5-Phosphate)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The proposed clinical study is intended to evaluate oral P5P for the treatment of patients confirmed to have Pyridox(am)ine 5'-Phosphate Oxidase (PNPO) deficiency via genetic analysis. There is an unmet clinical need for pharmaceutical grade P5P, as to date none has been made commercially available. Patients will receive pharmaceutical grade P5P according to their normal oral P5P dosing regimen, a...
Summary: This clinical trial aims to explore the effect of Vitamin B6 supplementation on anxiety sensory hyperreactivity in autistic adults. Researchers will compare a placebo group to high-dose Vitamin-B6 to see if vitamin B6 reduce anxiety and sensory reactivity differences in autism.
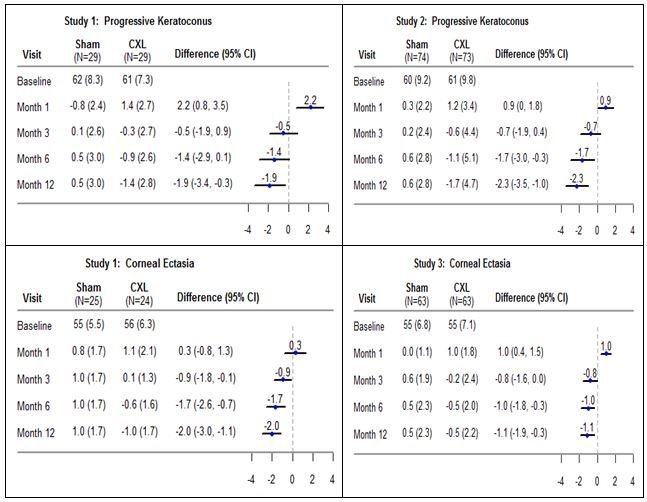
Summary: The objectives of this post market registry are to evaluate the safety and durability of treatment effect up to 3 years following cross-linking performed with Photrexa Viscous (riboflavin 5'- phosphate in 20% dextran ophthalmic solution), Photrexa (riboflavin 5'- phosphate ophthalmic solution), and the KXL System in patients with corneal ectasia following refractive surgery.
Related Latest Advances
Brand Information
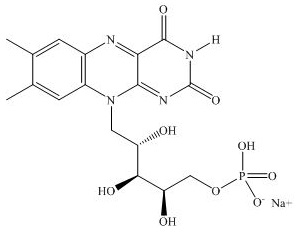
- PHOTREXA VISCOUS in a 3 mL glass syringe containing sterile 1.56 mg/mL riboflavin 5’-phosphate in 20% dextran ophthalmic solution for topical administration.
- PHOTREXA in a 3 mL glass syringe containing sterile 1.46 mg/mL riboflavin 5’-phosphate ophthalmic solution for topical administration.
- Patients should be advised not to rub their eyes for the first five days after their procedure.
- Patients may be sensitive to light and have a foreign body sensation. Patients should be advised that there may be discomfort in the treated eye and that sunglasses may help with light sensitivity.
- If patients experience severe pain in the eye or any sudden decrease in their vision, they should be advised to contact their physician immediately.
- If the bandage contact lens that was placed on the patient’s eye on the day of treatment falls out or becomes dislodged, the patient should be advised not to replace it and to contact their physician immediately.






