Brand Name
Etopophos
Generic Name
Etoposide
View Brand Information FDA approval date: May 01, 1996
Classification: Topoisomerase Inhibitor
Form: Injection, Capsule
What is Etopophos (Etoposide)?
ETOPOPHOS is a topoisomerase inhibitor indicated for the treatment of patients with: Refractory testicular tumors, in combination with other chemotherapeutic drugs. Small cell lung cancer, in combination with cisplatin, as first-line treatment.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
ETOPOPHOS (etoposide phosphate)
1DOSAGE FORMS AND STRENGTHS
For injection: 114 mg etoposide phosphate (equivalent to 100 mg etoposide), white to off-white, lyophilized powder in single-dose vial for reconstitution
2CONTRAINDICATIONS
ETOPOPHOS is contraindicated in patients with a history of a severe hypersensitivity reaction to etoposide products
3ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Myelosuppression
- Secondary leukemias
- Hypersensitivity reactions
3.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates, observed in the clinical trials of a drug, cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
ETOPOPHOS has been used as a single agent in clinical studies involving 206 patients with a variety of malignancies (including one non-Hodgkin’s lymphoma) and in combination with cisplatin in 60 patients with small cell lung cancer. The most common adverse reaction was neutropenia.
Other Important Adverse Reactions
Gastrointestinal Toxicity
Nausea and vomiting are the major gastrointestinal toxicities. The severity of nausea and vomiting is generally mild to moderate, with treatment discontinuation required in 1% of patients. Nausea and vomiting are managed with standard antiemetic therapy.
Other Toxicities
Other clinically important adverse reactions in clinical trials were:
Gastrointestinal: abdominal pain, constipation, dysphagia
General: fever
Ocular: transient cortical blindness, optic neuritis
Respiratory: interstitial pneumonitis/pulmonary fibrosis
Skin: pigmentation, radiation recall dermatitis, Stevens-Johnson syndrome, and toxic epidermal necrolysis
Neurologic: seizure, aftertaste
Hepatobiliary disorder: hepatotoxicity
Other clinically important adverse reactions in clinical trials were:
Gastrointestinal: abdominal pain, constipation, dysphagia
General: fever
Ocular: transient cortical blindness, optic neuritis
Respiratory: interstitial pneumonitis/pulmonary fibrosis
Skin: pigmentation, radiation recall dermatitis, Stevens-Johnson syndrome, and toxic epidermal necrolysis
Neurologic: seizure, aftertaste
Hepatobiliary disorder: hepatotoxicity
3.2Postmarketing Experience
The following adverse reactions have been identified during postapproval use of ETOPOPHOS. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Extravasation
Extravasation, resulting in local soft tissue toxicity. Extravasation of ETOPOPHOS can result in swelling, pain, cellulitis, and necrosis, including skin necrosis.
Extravasation, resulting in local soft tissue toxicity. Extravasation of ETOPOPHOS can result in swelling, pain, cellulitis, and necrosis, including skin necrosis.
Acute Renal Failure
Reversible cases of acute renal failure have been reported with administration of high dose (2220 mg/m2) ETOPOPHOS with total body irradiation used for hematopoietic stem cell transplantation. The ETOPOPHOS formulation contains dextran 40, which has been associated with acute renal failure when administered in high doses.
Reversible cases of acute renal failure have been reported with administration of high dose (2220 mg/m2) ETOPOPHOS with total body irradiation used for hematopoietic stem cell transplantation. The ETOPOPHOS formulation contains dextran 40, which has been associated with acute renal failure when administered in high doses.
4DRUG INTERACTIONS
Warfarin: Co-administration of ETOPOPHOS with warfarin can result in elevated international normalized ratio (INR). Measure INR frequently.
5OVERDOSAGE
No antidote has been established for ETOPOPHOS overdosage in humans. Based on animal studies, overdosage may result in neurotoxicity.
6DESCRIPTION
ETOPOPHOS (etoposide phosphate) is a topoisomerase inhibitor. The chemical name for etoposide phosphate is: 4'-Demethylepipodophyllotoxin 9-[4,6-O-(R)-ethylidene-β-D-glucopyranoside], 4' (dihydrogen phosphate).
Etoposide phosphate has the following structure:
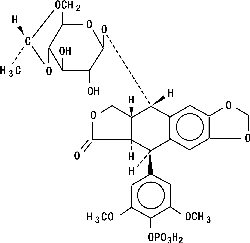
Etoposide phosphate is a phosphate ester of etoposide, a semi-synthetic derivative of podophyllotoxin. ETOPOPHOS is available for intravenous infusion as a sterile lyophilized powder in single-dose vials for reconstitution containing 114 mg etoposide phosphate, equivalent to 100 mg etoposide, 32.7 mg sodium citrate USP, and 300 mg dextran 40.
7CLINICAL STUDIES
Study 1 was a multicenter trial in patients, with previously untreated, small cell lung cancer, randomized (1:1) to receive either etoposide phosphate (80 mg/m
8REFERENCES
1. "OSHA Hazardous Drugs."
9HOW SUPPLIED/STORAGE AND HANDLING
How Supplied/Storage
ETOPOPHOS is supplied as a single-dose vial containing etoposide phosphate equivalent to 100 mg etoposide as a lyophilized powder for reconstitution, individually packaged in a carton:
NDC 61269-410-20
Store unopened vials at 2° to 8°C (36°-46°F). Keep vial in outer carton to protect from light.
ETOPOPHOS is supplied as a single-dose vial containing etoposide phosphate equivalent to 100 mg etoposide as a lyophilized powder for reconstitution, individually packaged in a carton:
NDC 61269-410-20
Store unopened vials at 2° to 8°C (36°-46°F). Keep vial in outer carton to protect from light.
Handling
ETOPOPHOS is a cytotoxic drug. Follow applicable special handling and disposal procedures.1
ETOPOPHOS is a cytotoxic drug. Follow applicable special handling and disposal procedures.1
10PATIENT COUNSELING INFORMATION
Myelosuppression
- Advise patients that periodic monitoring of their blood counts is required. Advise patients to contact their healthcare provider for new onset of bleeding, fever, or symptoms of infection
Embryo-Fetal Toxicity
- Advise females of reproductive potential of the potential risk to a fetus and to inform their healthcare provider of a known or suspected pregnancy
- Advise females of reproductive potential to use effective contraception during and 6 months after treatment with ETOPOPHOS
- Advise males with female sexual partners of reproductive potential to use condoms during treatment with ETOPOPHOS and for at least 4 months after the final dose
Licensed by:
Product of Germany
Distributed by:
H2-Pharma, LLC
Montgomery, AL 36117