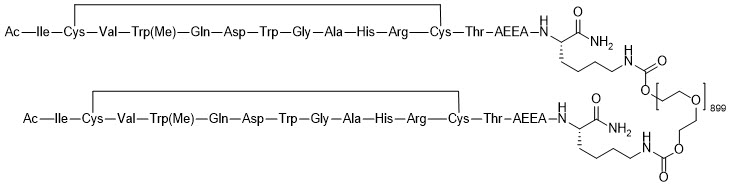
Pegcetacoplan
What is Empaveli (Pegcetacoplan)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The main purpose of this study is to assess the ocular and systemic safety and tolerability of RO7669330 in participants with GA secondary to AMD in at least one eye in Part 1, or both eyes in Part 2, after multiple unilateral intravitreal (IVT) doses.
Summary: Age-related macular degeneration (AMD) is the abnormal growth of new blood vessels in the light-sensitive tissue at the back of the eye called the retina. Geographic Atrophy (GA) is an advanced form of dry AMD. The purpose of this study is to assess the adverse events and how intravitreal ABBV-6628 moves through the body of adult participants with secondary to age-related macular degeneration ABBV...
Summary: A Phase 2, Randomized, Placebo-controlled, Multicenter, Masked Study to Evaluate the Efficacy, Safety, Tolerability, and Pharmacodynamics of Multidose APL-3007 in Combination with Syfovre/Pegcetacoplan (APL-2) in Patients Diagnosed with Geographic Atrophy Secondary to Age-Related Macular Degeneration
Related Latest Advances
Brand Information
- Complete or update vaccination for encapsulated bacteria at least 2 weeks prior to the first dose of EMPAVELI, unless the risks of delaying therapy with EMPAVELI outweigh the risk of developing a serious infection. Comply with the most current Advisory Committee on Immunization Practices (ACIP) recommendations for vaccinations against encapsulated bacteria in patients receiving a complement inhibitor.
- Patients receiving EMPAVELI are at increased risk for invasive disease caused by encapsulated bacteria, even if they develop antibodies following vaccination. Monitor patients for early signs and symptoms of serious infections and evaluate immediately if infection is suspected.
- in patients with hypersensitivity to pegcetacoplan or to any of the excipients
- for initiation in patients with unresolved serious infection caused by encapsulated bacteria including
- Serious Infections Caused by Encapsulated Bacteria
- Infusion-Related Reactions
- Anaphylaxis and urticaria